Page 220 - Advanced Thermodynamics for Engineers, Second Edition
P. 220
208 CHAPTER 10 THERMODYNAMICS OF COMBUSTION
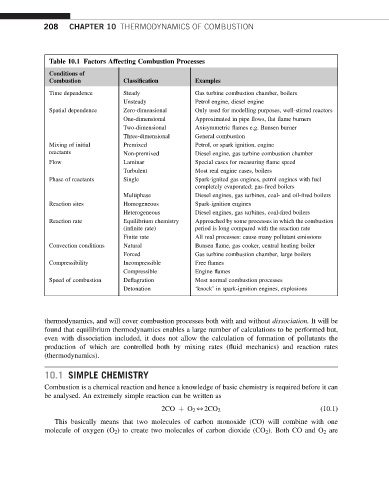
Table 10.1 Factors Affecting Combustion Processes
Conditions of
Combustion Classification Examples
Time dependence Steady Gas turbine combustion chamber, boilers
Unsteady Petrol engine, diesel engine
Spatial dependence Zero-dimensional Only used for modelling purposes, well-stirred reactors
One-dimensional Approximated in pipe flows, flat flame burners
Two-dimensional Axisymmetric flames e.g. Bunsen burner
Three-dimensional General combustion
Mixing of initial Premixed Petrol, or spark ignition, engine
reactants Non-premixed Diesel engine, gas turbine combustion chamber
Flow Laminar Special cases for measuring flame speed
Turbulent Most real engine cases, boilers
Phase of reactants Single Spark-ignited gas engines, petrol engines with fuel
completely evaporated; gas-fired boilers
Multiphase Diesel engines, gas turbines, coal- and oil-fired boilers
Reaction sites Homogeneous Spark-ignition engines
Heterogeneous Diesel engines, gas turbines, coal-fired boilers
Reaction rate Equilibrium chemistry Approached by some processes in which the combustion
(infinite rate) period is long compared with the reaction rate
Finite rate All real processes: cause many pollutant emissions
Convection conditions Natural Bunsen flame, gas cooker, central heating boiler
Forced Gas turbine combustion chamber, large boilers
Compressibility Incompressible Free flames
Compressible Engine flames
Speed of combustion Deflagration Most normal combustion processes
Detonation ‘knock’ in spark-ignition engines, explosions
thermodynamics, and will cover combustion processes both with and without dissociation. It will be
found that equilibrium thermodynamics enables a large number of calculations to be performed but,
even with dissociation included, it does not allow the calculation of formation of pollutants the
production of which are controlled both by mixing rates (fluid mechanics) and reaction rates
(thermodynamics).
10.1 SIMPLE CHEMISTRY
Combustion is a chemical reaction and hence a knowledge of basic chemistry is required before it can
be analysed. An extremely simple reaction can be written as
(10.1)
2CO þ O 2 52CO 2
This basically means that two molecules of carbon monoxide (CO) will combine with one
molecule of oxygen (O 2 ) to create two molecules of carbon dioxide (CO 2 ). Both CO and O 2 are