Page 227 - Advanced Thermodynamics for Engineers, Second Edition
P. 227
10.4 APPLICATION OF THE ENERGY EQUATION 215
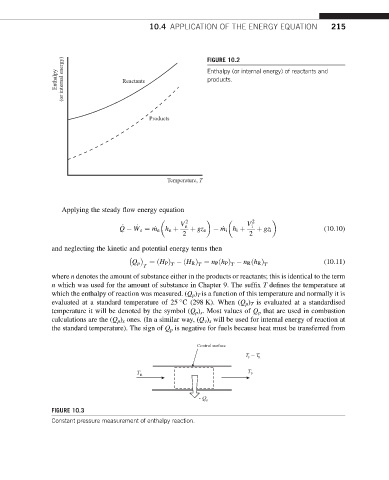
Enthalpy (or internal energy) of reactants and
Enthalpy (or internal energy) Reactants FIGURE 10.2
products.
Products
Temperature, T
Applying the steady flow energy equation
2 2
_ _ V e V i
2 2
Q W s ¼ _ m e h e þ þ gz e _ m i h i þ þ gz i (10.10)
and neglecting the kinetic and potential energy terms then
Q p (10.11)
T
T
T
T ¼ðH P Þ ðH R Þ ¼ n P ðh P Þ n R ðh R Þ T
where n denotes the amount of substance either in the products or reactants; this is identical to the term
n which was used for the amount of substance in Chapter 9. The suffix T defines the temperature at
which the enthalpy of reaction was measured. (Q p ) T is a function of this temperature and normally it is
evaluated at a standard temperature of 25 C (298 K). When (Q p ) T is evaluated at a standardised
temperature it will be denoted by the symbol (Q p ) s . Most values of Q p that are used in combustion
calculations are the (Q p ) s ones. (In a similar way, (Q v ) s will be used for internal energy of reaction at
the standard temperature). The sign of Q p is negative for fuels because heat must be transferred from
Control surface
T = T R
P
T P
T R
- Q p
FIGURE 10.3
Constant pressure measurement of enthalpy reaction.