Page 233 - Advanced Thermodynamics for Engineers, Second Edition
P. 233
10.5 COMBUSTION PROCESSES 221
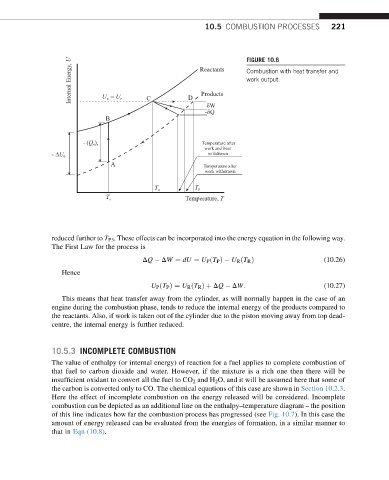
Internal Energy, U Reactants FIGURE 10.6
Combustion with heat transfer and
work output.
Products
D
C
U = U P
R
W
-Q
B
- (Q ) Temperature after
vs
work and heat
withdrawn
- U 0
A Temperature after
work withdrawn
T R T P
Temperature, T
T s
reduced further to T P3 . These effects can be incorporated into the energy equation in the following way.
The First Law for the process is
(10.26)
DQ DW ¼ dU ¼ U P ðT P Þ U R ðT R Þ
Hence
U P ðT P Þ¼ U R ðT R Þþ DQ DW: (10.27)
This means that heat transfer away from the cylinder, as will normally happen in the case of an
engine during the combustion phase, tends to reduce the internal energy of the products compared to
the reactants. Also, if work is taken out of the cylinder due to the piston moving away from top dead-
centre, the internal energy is further reduced.
10.5.3 INCOMPLETE COMBUSTION
The value of enthalpy (or internal energy) of reaction for a fuel applies to complete combustion of
that fuel to carbon dioxide and water. However, if the mixture is a rich one then there will be
insufficient oxidant to convert all the fuel to CO 2 and H 2 O, and it will be assumed here that some of
the carbon is converted only to CO. The chemical equations of this case are shown in Section 10.2.3.
Here the effect of incomplete combustion on the energy released will be considered. Incomplete
combustion can be depicted as an additional line on the enthalpy–temperature diagram – the position
of this line indicates how far the combustion process has progressed (see Fig. 10.7). In this case the
amount of energy released can be evaluated from the energies of formation, in a similar manner to
that in Eqn (10.8).