Page 271 - Advanced Thermodynamics for Engineers, Second Edition
P. 271
260 CHAPTER 12 CHEMICAL EQUILIBRIUM AND DISSOCIATION
P
2
Standard Gibbs Energy
of mixing of A and B
R
a +
o
o
1 G -μ o c (1-ε)[μ o μ −2μ ]
b
c
Normalised Gibbs Energy 0 S Q
ε
ε
T 2RT[(1- )ln((1- )/2)+ε lnε]
-1
Minimum
Gibbs Energy
of mixture
-2
0 0.5 1.0
Fraction of reaction, ε
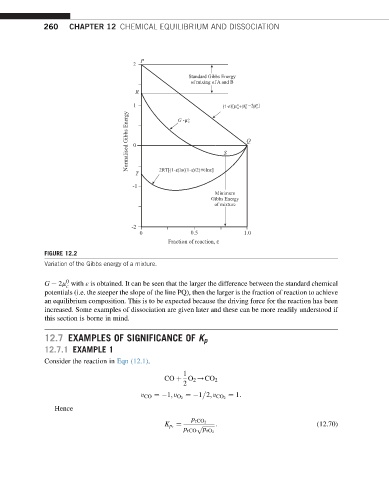
FIGURE 12.2
Variation of the Gibbs energy of a mixture.
0
G 2m with ε is obtained. It can be seen that the larger the difference between the standard chemical
c
potentials (i.e. the steeper the slope of the line PQ), then the larger is the fraction of reaction to achieve
an equilibrium composition. This is to be expected because the driving force for the reaction has been
increased. Some examples of dissociation are given later and these can be more readily understood if
this section is borne in mind.
12.7 EXAMPLES OF SIGNIFICANCE OF K p
12.7.1 EXAMPLE 1
Consider the reaction in Eqn (12.1).
1
CO þ O 2 /CO 2
2
¼ 1:
y CO ¼ 1; y O 2 ¼ 1 2; y CO 2
Hence
p rCO 2
ffiffiffiffiffiffiffiffi : (12.70)
K p r ¼
p
p rCO p rO 2