Page 318 - Advanced Thermodynamics for Engineers, Second Edition
P. 318
308 CHAPTER 14 CHEMICAL KINETICS
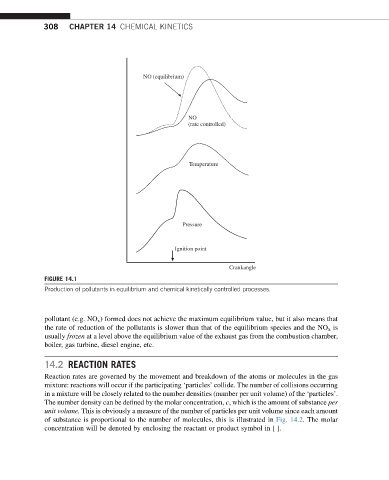
NO (equilibrium)
NO
(rate controlled)
Temperature
Pressure
Ignition point
Crankangle
FIGURE 14.1
Production of pollutants in equilibrium and chemical kinetically controlled processes.
pollutant (e.g. NO x ) formed does not achieve the maximum equilibrium value, but it also means that
the rate of reduction of the pollutants is slower than that of the equilibrium species and the NO x is
usually frozen at a level above the equilibrium value of the exhaust gas from the combustion chamber,
boiler, gas turbine, diesel engine, etc.
14.2 REACTION RATES
Reaction rates are governed by the movement and breakdown of the atoms or molecules in the gas
mixture: reactions will occur if the participating ‘particles’ collide. The number of collisions occurring
in a mixture will be closely related to the number densities (number per unit volume) of the ‘particles’.
The number density can be defined by the molar concentration, c, which is the amount of substance per
unit volume. This is obviously a measure of the number of particles per unit volume since each amount
of substance is proportional to the number of molecules, this is illustrated in Fig. 14.2. The molar
concentration will be denoted by enclosing the reactant or product symbol in [ ].