Page 168 - Advanced thermodynamics for engineers
P. 168
154 CHAPTER 7 GENERAL THERMODYNAMIC RELATIONSHIPS
Pressure, p
Critical point
Vapour
Liquid + vapour
Liquid
T 1
T C
T 2
T 3
Specific volume, v
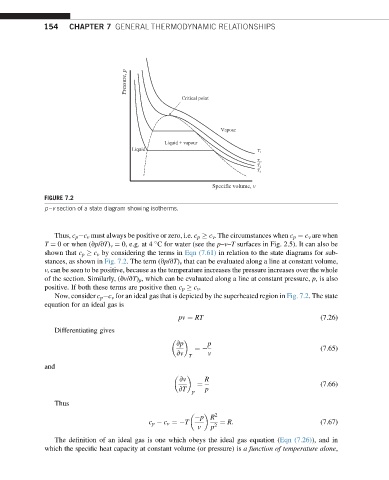
FIGURE 7.2
p –v section of a state diagram showing isotherms.
Thus, c p c v must always be positive or zero, i.e. c p c v . The circumstances when c p ¼ c v are when
T ¼ 0orwhen(vp/vT) v ¼ 0, e.g. at 4 C for water (see the p–v–T surfaces in Fig. 2.5). It can also be
shown that c p c v by considering the terms in Eqn (7.61) in relation to the state diagrams for sub-
stances, as shown in Fig. 7.2. The term (vp/vT) v that can be evaluated along a line at constant volume,
v, can be seen to be positive, because as the temperature increases the pressure increases over the whole
of the section. Similarly, (vv/vT) p , which can be evaluated along a line at constant pressure, p, is also
positive. If both these terms are positive then c p c v .
Now, consider c p c v for an ideal gas that is depicted by the superheated region in Fig. 7.2. The state
equation for an ideal gas is
pv ¼ RT (7.26)
Differentiating gives
vp p
¼ (7.65)
vv v
T
and
vv R
¼ (7.66)
vT p
p
Thus
2
p R
c p c v ¼ T ¼ R: (7.67)
v p 2
The definition of an ideal gas is one which obeys the ideal gas equation (Eqn (7.26)), and in
which the specific heat capacity at constant volume (or pressure) is a function of temperature alone,