Page 250 - Advanced thermodynamics for engineers
P. 250
11.2 ENERGY OF FORMATION 237
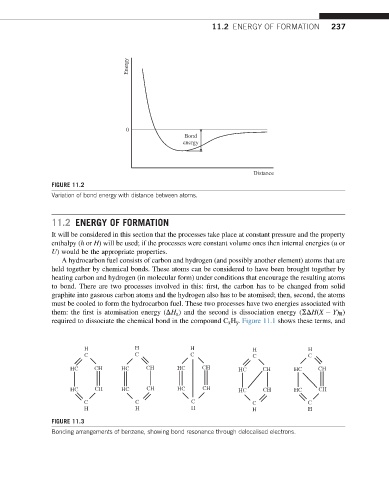
Energy
0
Bond
energy
Distance
FIGURE 11.2
Variation of bond energy with distance between atoms.
11.2 ENERGY OF FORMATION
It will be considered in this section that the processes take place at constant pressure and the property
enthalpy (h or H) will be used; if the processes were constant volume ones then internal energies (u or
U) would be the appropriate properties.
A hydrocarbon fuel consists of carbon and hydrogen (and possibly another element) atoms that are
held together by chemical bonds. These atoms can be considered to have been brought together by
heating carbon and hydrogen (in molecular form) under conditions that encourage the resulting atoms
to bond. There are two processes involved in this: first, the carbon has to be changed from solid
graphite into gaseous carbon atoms and the hydrogen also has to be atomised; then, second, the atoms
must be cooled to form the hydrocarbon fuel. These two processes have two energies associated with
them: the first is atomisation energy (DH a ) and the second is dissociation energy (SDH(X Y) R )
required to dissociate the chemical bond in the compound C x H y . Figure 11.1 shows these terms, and
H H H H H
C C C C C
HC CH HC CH HC CH HC CH HC CH
HC CH HC CH HC CH HC CH HC CH
C C C C C
H H H H H
FIGURE 11.3
Bonding arrangements of benzene, showing bond resonance through delocalised electrons.