Page 278 - Advanced thermodynamics for engineers
P. 278
266 CHAPTER 12 CHEMICAL EQUILIBRIUM AND DISSOCIATION
290000
288000
286000
Energy released from
Energy in products / (kJ) 282000
dissociation equation
284000
280000
278000
276000
Energy absorbed by gases
274000
272000
2900 2910 2920 2930 9 2 4 0 5 9 2 0 2960 2970 2 8 9 0 2 9 0 9 0 0 3 0
Temperature, T / (K)
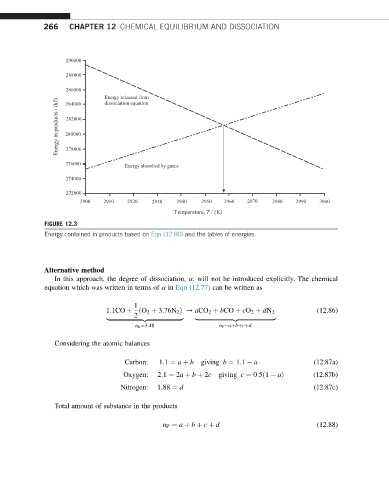
FIGURE 12.3
Energy contained in products based on Eqn (12.80) and the tables of energies.
Alternative method
In this approach, the degree of dissociation, a, will not be introduced explicitly. The chemical
equation which was written in terms of a in Eqn (12.77) can be written as
1
1:1CO þ ðO 2 þ 3:76N 2 Þ / aCO 2 þ bCO þ cO 2 þ dN 2 (12.86)
2
|fflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflffl{zfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflffl} |fflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflffl{zfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflffl}
n R ¼3:48 n P ¼aþbþcþd
Considering the atomic balances
Carbon: 1:1 ¼ a þ b giving b ¼ 1:1 a (12.87a)
Oxygen: 2:1 ¼ 2a þ b þ 2c giving c ¼ 0:5ð1 aÞ (12.87b)
Nitrogen: 1:88 ¼ d (12.87c)
Total amount of substance in the products
n P ¼ a þ b þ c þ d (12.88)