Page 313 - Advanced thermodynamics for engineers
P. 313
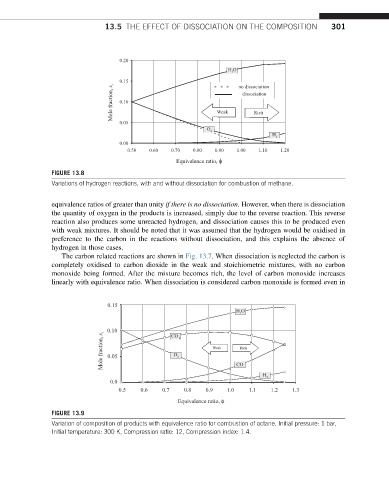
13.5 THE EFFECT OF DISSOCIATION ON THE COMPOSITION 301
0.20
HO
0.15 no dissociation
Mole fraction, x 0.10 Weak dissociation
Rich
0.05
O
H
0.00
0.50 0.60 0.70 0.80 0.90 1.00 1.10 1.20
Equivalence ratio, φ
FIGURE 13.8
Variations of hydrogen reactions, with and without dissociation for combustion of methane.
equivalence ratios of greater than unity if there is no dissociation. However, when there is dissociation
the quantity of oxygen in the products is increased, simply due to the reverse reaction. This reverse
reaction also produces some unreacted hydrogen, and dissociation causes this to be produced even
with weak mixtures. It should be noted that it was assumed that the hydrogen would be oxidised in
preference to the carbon in the reactions without dissociation, and this explains the absence of
hydrogen in those cases.
The carbon related reactions are shown in Fig. 13.7. When dissociation is neglected the carbon is
completely oxidised to carbon dioxide in the weak and stoichiometric mixtures, with no carbon
monoxide being formed. After the mixture becomes rich, the level of carbon monoxide increases
linearly with equivalence ratio. When dissociation is considered carbon monoxide is formed even in
1.50E-01
0.15
HO
Mole fraction, x 0.10 CO Weak Rich
1.00E-01
O
0.05
5.00E-02
CO
H
0.0
0.00E+00
1
0.5 0.6 0.7 0.8 0.9 1.0 1.1 1.2 1.3
1.3
1.1
1.2
0.7
0.6
0.5
0.9
0.8
Equivalence ratio, φ
FIGURE 13.9
Variation of composition of products with equivalence ratio for combustion of octane. Initial pressure: 1 bar,
Initial temperature: 300 K, Compression ratio: 12, Compression index: 1.4.