Page 316 - Advanced thermodynamics for engineers
P. 316
304 CHAPTER 13 EFFECT OF DISSOCIATION ON COMBUSTION PARAMETERS
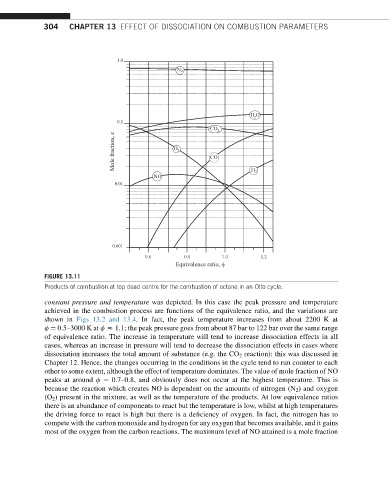
1.0
N 2
H O
2
0.1
CO 2
Mole fraction, x O 2 CO
NO H 2
0.01
0.001
0.6 0.8 1.0 1.2
Equivalence ratio, φ
FIGURE 13.11
Products of combustion at top dead centre for the combustion of octane in an Otto cycle.
constant pressure and temperature was depicted. In this case the peak pressure and temperature
achieved in the combustion process are functions of the equivalence ratio, and the variations are
shown in Figs 13.2 and 13.4. In fact, the peak temperature increases from about 2200 K at
f ¼ 0.5–3000 K at f z 1.1; the peak pressure goes from about 87 bar to 122 bar over the same range
of equivalence ratio. The increase in temperature willtendtoincreasedissociationeffectsinall
cases, whereas an increase in pressure will tend to decrease the dissociation effects in cases where
dissociation increases the total amount of substance (e.g. the CO 2 reaction): this was discussed in
Chapter 12. Hence, the changes occurring in the conditions in the cycle tend to run counter to each
other to some extent, although the effect of temperature dominates. The value of mole fraction of NO
peaks at around f ¼ 0.7–0.8, and obviously does not occur at the highest temperature. This is
because the reaction which creates NO is dependent on the amounts of nitrogen (N 2 ) and oxygen
(O 2 ) present in the mixture, as well as the temperature of the products. At low equivalence ratios
there is an abundance of components to react but the temperature is low, whilst at high temperatures
thedriving forcetoreact is high butthere is adeficiency of oxygen. In fact, the nitrogen has to
compete with the carbon monoxide and hydrogen for any oxygen that becomes available, and it gains
most of the oxygen from the carbon reactions. The maximum level of NO attained is a mole fraction