Page 323 - Advanced thermodynamics for engineers
P. 323
312 CHAPTER 14 CHEMICAL KINETICS
lnk
1/ T
FIGURE 14.3
Relationship between reaction rate and temperature.
related to the temperature in those cases. The term E in the exponent is referred to as the activation
energy. The values of A and E are dependent on the reactions being considered, and some values are
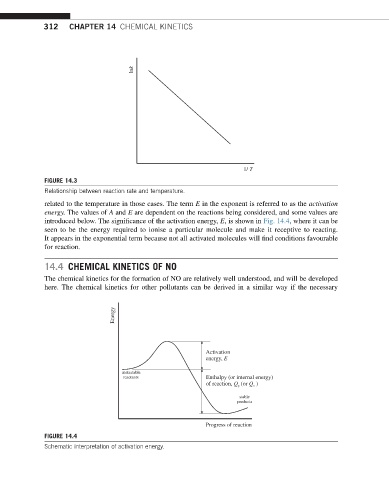
introduced below. The significance of the activation energy, E, is shown in Fig. 14.4, where it can be
seen to be the energy required to ionise a particular molecule and make it receptive to reacting.
It appears in the exponential term because not all activated molecules will find conditions favourable
for reaction.
14.4 CHEMICAL KINETICS OF NO
The chemical kinetics for the formation of NO are relatively well understood, and will be developed
here. The chemical kinetics for other pollutants can be derived in a similar way if the necessary
Energy
Activation
energy, E
metastable
reactants Enthalpy (or internal energy)
of reaction, Q (or Q )
stable
products
Progress of reaction
FIGURE 14.4
Schematic interpretation of activation energy.