Page 325 - Advances in Eco-Fuels for a Sustainable Environment
P. 325
Thermal depolymerization of biogas digestate 281
reactions [22]. Thus, nitrogenous compounds present in the digestate are not oxidized
for NO and N 2 O gas formation but rather serve to enrich the nitrogen content of the HTL
biochar product or contribute to the gaseous product yield in the form of gaseous N 2
[23]. The nitrous oxide gases of NO and N 2 O from the uncontrolled oxidation of nitrog-
enous compounds present in the digestate are recognized as almost 300 times worse than
CO 2 , when compared on the global warming potential metric scale [24].Thisimplies
that in the absence of the generation of toxic HTL gas components, the CO 2 gas gen-
erated from the HTL process will present a lower unfavorable environmental impact
than possible unfavorable environmental impacts that may be observed from the direct
application of digestate on agricultural soils. These observations therefore further rein-
force the suitability of the proposed alternative application of HTL based one-step
digestate processing technology.
As stated briefly in the discussions provided above, hydrothermal conversion tech-
nologies convert wet biomass to useful products under conditions of high pressure and
high temperatures. Under these conditions, crucial pyrolytic reactions are initiated and
promoted [25, 26]. During hydrothermal conversion reactions, the presence of water is
recognized as a necessary requirement because the water performs the role of a cat-
alyst and also serves as a much needed reaction medium. The major products from the
hydrothermal processing will vary significantly, depending on the reaction conditions
imposed, ranging from subcritical to supercritical water conditions [27]. The major
products obtainable from the hydrothermal processing of biomass under different
reaction conditions are summarized in Table 10.1.
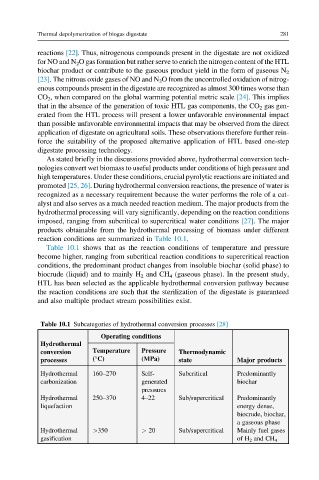
Table 10.1 shows that as the reaction conditions of temperature and pressure
become higher, ranging from subcritical reaction conditions to supercritical reaction
conditions, the predominant product changes from insoluble biochar (solid phase) to
biocrude (liquid) and to mainly H 2 and CH 4 (gaseous phase). In the present study,
HTL has been selected as the applicable hydrothermal conversion pathway because
the reaction conditions are such that the sterilization of the digestate is guaranteed
and also multiple product stream possibilities exist.
Table 10.1 Subcategories of hydrothermal conversion processes [28]
Operating conditions
Hydrothermal
Temperature Pressure
conversion Thermodynamic
(°C) (MPa)
processes state Major products
Hydrothermal 160–270 Self- Subcritical Predominantly
carbonization generated biochar
pressures
Hydrothermal 250–370 4–22 Sub/supercritical Predominantly
liquefaction energy dense,
biocrude, biochar,
a gaseous phase
Hydrothermal >350 > 20 Sub/supercritical Mainly fuel gases
gasification of H 2 and CH 4