Page 368 - Advances in Eco-Fuels for a Sustainable Environment
P. 368
Physicochemical fuel properties and tribological behavior of aegle marmelos correa biodiesel 323
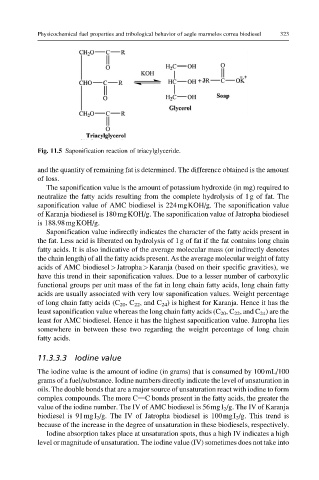
Fig. 11.5 Saponification reaction of triacylglyceride.
and the quantity of remaining fat is determined. The difference obtained is the amount
of loss.
The saponification value is the amount of potassium hydroxide (in mg) required to
neutralize the fatty acids resulting from the complete hydrolysis of 1g of fat. The
saponification value of AMC biodiesel is 224mgKOH/g. The saponification value
of Karanja biodiesel is 180mgKOH/g. The saponification value of Jatropha biodiesel
is 188.98mgKOH/g.
Saponification value indirectly indicates the character of the fatty acids present in
the fat. Less acid is liberated on hydrolysis of 1g of fat if the fat contains long chain
fatty acids. It is also indicative of the average molecular mass (or indirectly denotes
the chain length) of all the fatty acids present. As the average molecular weight of fatty
acids of AMC biodiesel>Jatropha>Karanja (based on their specific gravities), we
have this trend in their saponification values. Due to a lesser number of carboxylic
functional groups per unit mass of the fat in long chain fatty acids, long chain fatty
acids are usually associated with very low saponification values. Weight percentage
of long chain fatty acids (C 20 ,C 22 , and C 24 ) is highest for Karanja. Hence it has the
least saponification value whereas the long chain fatty acids (C 20 ,C 22 , and C 24 ) are the
least for AMC biodiesel. Hence it has the highest saponification value. Jatropha lies
somewhere in between these two regarding the weight percentage of long chain
fatty acids.
11.3.3.3 Iodine value
The iodine value is the amount of iodine (in grams) that is consumed by 100mL/100
grams of a fuel/substance. Iodine numbers directly indicate the level of unsaturation in
oils. The double bonds that are a major source of unsaturation react with iodine to form
complex compounds. The more C]C bonds present in the fatty acids, the greater the
value of the iodine number. The IV of AMC biodiesel is 56mgI 2 /g. The IV of Karanja
biodiesel is 91mgI 2 /g. The IV of Jatropha biodiesel is 100mgI 2 /g. This trend is
because of the increase in the degree of unsaturation in these biodiesels, respectively.
Iodine absorption takes place at unsaturation spots, thus a high IV indicates a high
level or magnitude of unsaturation. The iodine value (IV) sometimes does not take into