Page 260 - Advances in Forensic Applications of Mass Spectrometry - Jehuda Yinon
P. 260
1522_book.fm Page 233 Thursday, November 13, 2003 9:58 AM
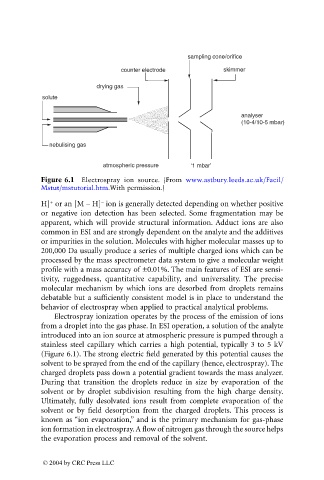
sampling cone/orifice
counter electrode skimmer
drying gas
solute
analyser
(10-4/10-5 mbar)
nebulising gas
atmospheric pressure ‘1 mbar’
Figure 6.1 Electrospray ion source. (From www.astbury.leeds.ac.uk/Facil/
Mstut/mstutorial.htm.With permission.)
+
–
H] or an [M – H] ion is generally detected depending on whether positive
or negative ion detection has been selected. Some fragmentation may be
apparent, which will provide structural information. Adduct ions are also
common in ESI and are strongly dependent on the analyte and the additives
or impurities in the solution. Molecules with higher molecular masses up to
200,000 Da usually produce a series of multiple charged ions which can be
processed by the mass spectrometer data system to give a molecular weight
profile with a mass accuracy of ±0.01%. The main features of ESI are sensi-
tivity, ruggedness, quantitative capability, and universality. The precise
molecular mechanism by which ions are desorbed from droplets remains
debatable but a sufficiently consistent model is in place to understand the
behavior of electrospray when applied to practical analytical problems.
Electrospray ionization operates by the process of the emission of ions
from a droplet into the gas phase. In ESI operation, a solution of the analyte
introduced into an ion source at atmospheric pressure is pumped through a
stainless steel capillary which carries a high potential, typically 3 to 5 kV
(Figure 6.1). The strong electric field generated by this potential causes the
solvent to be sprayed from the end of the capillary (hence, electrospray). The
charged droplets pass down a potential gradient towards the mass analyzer.
During that transition the droplets reduce in size by evaporation of the
solvent or by droplet subdivision resulting from the high charge density.
Ultimately, fully desolvated ions result from complete evaporation of the
solvent or by field desorption from the charged droplets. This process is
known as “ion evaporation,” and is the primary mechanism for gas-phase
ion formation in electrospray. A flow of nitrogen gas through the source helps
the evaporation process and removal of the solvent.
© 2004 by CRC Press LLC