Page 268 - Advances in Forensic Applications of Mass Spectrometry - Jehuda Yinon
P. 268
1522_book.fm Page 241 Thursday, November 13, 2003 9:58 AM
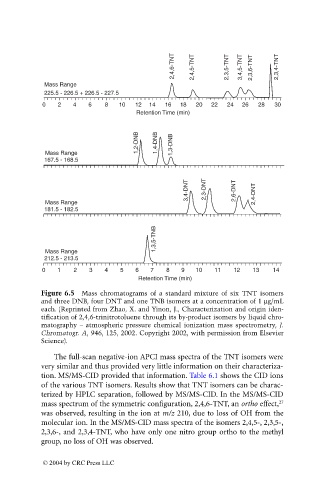
2,4,6-TNT 2,4,5-TNT 2,3,5-TNT 3,4,5-TNT 2,3,6-TNT 2,3,4-TNT
Mass Range
225.5 - 226.5 + 226.5 - 227.5
0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30
Retention Time (min)
1,2-DNB 1,4-DNB 1,3-DNB
Mass Range
167.5 - 168.5
3,4-DNT 2,3-DNT 2,6-DNT 2,4-DNT
Mass Range
181.5 - 182.5
1,3,5-TNB
Mass Range
212.5 - 213.5
0 1 2 3 4 5 6 7 8 9 10 11 12 13 14
Retention Time (min)
Figure 6.5 Mass chromatograms of a standard mixture of six TNT isomers
and three DNB, four DNT and one TNB isomers at a concentration of 1 mg/mL
each. (Reprinted from Zhao, X. and Yinon, J., Characterization and origin iden-
tification of 2,4,6-trinitrotoluene through its by-product isomers by liquid chro-
matography – atmospheric pressure chemical ionization mass spectrometry, J.
Chromatogr. A, 946, 125, 2002. Copyright 2002, with permission from Elsevier
Science).
The full-scan negative-ion APCI mass spectra of the TNT isomers were
very similar and thus provided very little information on their characteriza-
tion. MS/MS-CID provided that information. Table 6.1 shows the CID ions
of the various TNT isomers. Results show that TNT isomers can be charac-
terized by HPLC separation, followed by MS/MS-CID. In the MS/MS-CID
mass spectrum of the symmetric configuration, 2,4,6-TNT, an ortho effect, 27
was observed, resulting in the ion at m/z 210, due to loss of OH from the
molecular ion. In the MS/MS-CID mass spectra of the isomers 2,4,5-, 2,3,5-,
2,3,6-, and 2,3,4-TNT, who have only one nitro group ortho to the methyl
group, no loss of OH was observed.
© 2004 by CRC Press LLC