Page 276 - Advances in Forensic Applications of Mass Spectrometry - Jehuda Yinon
P. 276
1522_book.fm Page 249 Thursday, November 13, 2003 9:58 AM
-
[M* - H]
241.2
100
95
90
85
80
75
70 [M* - CH ] 2 -
Relative abundance 60
65
228.5
55
50
45
40
35
[M*-NO -H] -
30 2
195.3 211.6226.4 242.2
25
20 212.6 - [M*+NO +NO] -
15 253.2 [M - H] 318.1 2
182.0 257.1 286.1
10 224.8
5
0
60 80 100 120 140 160 180 200 220 240 260 280 300 320 340
m/z
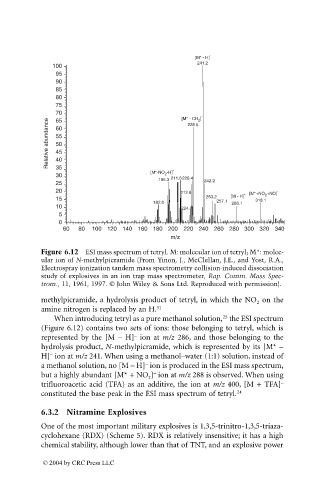
Figure 6.12 ESI mass spectrum of tetryl. M: molecular ion of tetryl; M*: molec-
ular ion of N-methylpicramide (From Yinon, J., McClellan, J.E., and Yost, R.A.,
Electrospray ionization tandem mass spectrometry collision-induced dissociation
study of explosives in an ion trap mass spectrometer, Rap. Comm. Mass Spec-
trom., 11, 1961, 1997. © John Wiley & Sons Ltd. Reproduced with permission).
methylpicramide, a hydrolysis product of tetryl, in which the NO on the
2
amine nitrogen is replaced by an H. 31
25
When introducing tetryl as a pure methanol solution, the ESI spectrum
(Figure 6.12) contains two sets of ions: those belonging to tetryl, which is
–
represented by the [M – H] ion at m/z 286, and those belonging to the
hydrolysis product, N-methylpicramide, which is represented by its [M* –
–
H] ion at m/z 241. When using a methanol–water (1:1) solution, instead of
–
a methanol solution, no [M – H] ion is produced in the ESI mass spectrum,
–
but a highly abundant [M* + NO ] ion at m/z 288 is observed. When using
2
trifluoroacetic acid (TFA) as an additive, the ion at m/z 400, [M + TFA] –
constituted the base peak in the ESI mass spectrum of tetryl. 24
6.3.2 Nitramine Explosives
One of the most important military explosives is 1,3,5-trinitro-1,3,5-triaza-
cyclohexane (RDX) (Scheme 5). RDX is relatively insensitive; it has a high
chemical stability, although lower than that of TNT, and an explosive power
© 2004 by CRC Press LLC