Page 102 - Advances in Textile Biotechnology
P. 102
Enzymatic hydrolysis and modifi cation of core polymer fi bres 83
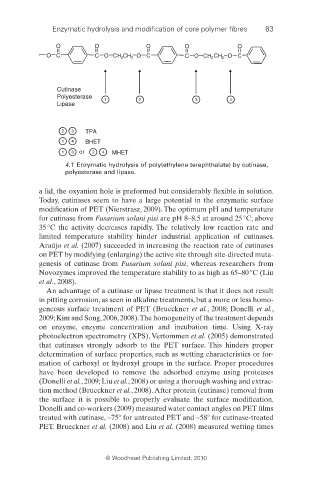
O O O O O
O C C O CH CH O C C O CH 2 O C
2 2 CH 2
Cutinase
Polyesterase
1 1 22 33 44
Lipase
2 3 TPA
1 4 BHET
1 3 or 2 4 MHET
4.1 Enzymatic hydrolysis of poly(ethylene terephthalate) by cutinase,
polyesterase and lipase.
a lid, the oxyanion hole is preformed but considerably flexible in solution.
Today, cutinases seem to have a large potential in the enzymatic surface
modifi cation of PET (Nierstrasz, 2009). The optimum pH and temperature
for cutinase from Fusarium solani pisi are pH 8–8.5 at around 25 °C; above
35 °C the activity decreases rapidly. The relatively low reaction rate and
limited temperature stability hinder industrial application of cutinases.
Araújo et al. (2007) succeeded in increasing the reaction rate of cutinases
on PET by modifying (enlarging) the active site through site-directed muta-
genesis of cutinase from Fusarium solani pisi, whereas researchers from
Novozymes improved the temperature stability to as high as 65–80 °C (Liu
et al., 2008).
An advantage of a cutinase or lipase treatment is that it does not result
in pitting corrosion, as seen in alkaline treatments, but a more or less homo-
geneous surface treatment of PET (Brueckner et al., 2008; Donelli et al.,
2009; Kim and Song, 2006, 2008). The homogeneity of the treatment depends
on enzyme, enzyme concentration and incubation time. Using X-ray
photoelectron spectrometry (XPS), Vertommen et al. (2005) demonstrated
that cutinases strongly adsorb to the PET surface. This hinders proper
determination of surface properties, such as wetting characteristics or for-
mation of carboxyl or hydroxyl groups in the surface. Proper procedures
have been developed to remove the adsorbed enzyme using proteases
(Donelli et al., 2009; Liu et al., 2008) or using a thorough washing and extrac-
tion method (Brueckner et al., 2008). After protein (cutinase) removal from
the surface it is possible to properly evaluate the surface modifi cation.
Donelli and co-workers (2009) measured water contact angles on PET fi lms
treated with cutinase, ∼75° for untreated PET and ∼58° for cutinase-treated
PET. Brueckner et al. (2008) and Liu et al. (2008) measured wetting times
© Woodhead Publishing Limited, 2010