Page 106 - Advances in Textile Biotechnology
P. 106
Enzymatic hydrolysis and modifi cation of core polymer fi bres 87
O
H
R N R 1
NH N
H
O O
Protease
Amidase
O Cutinase
R OH H N R1
2
NH N
H
O O
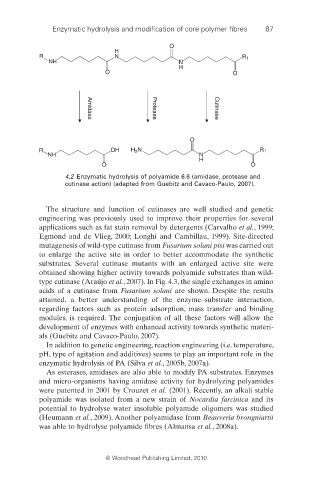
4.2 Enzymatic hydrolysis of polyamide 6.6 (amidase, protease and
cutinase action) (adapted from Guebitz and Cavaco-Paulo, 2007).
The structure and function of cutinases are well studied and genetic
engineering was previously used to improve their properties for several
applications such as fat stain removal by detergents (Carvalho et al., 1999;
Egmond and de Vlieg, 2000; Longhi and Cambillau, 1999). Site-directed
mutagenesis of wild-type cutinase from Fusarium solani pisi was carried out
to enlarge the active site in order to better accommodate the synthetic
substrates. Several cutinase mutants with an enlarged active site were
obtained showing higher activity towards polyamide substrates than wild-
type cutinase (Araújo et al., 2007). In Fig. 4.3, the single exchanges in amino
acids of a cutinase from Fusarium solani are shown. Despite the results
attained, a better understanding of the enzyme–substrate interaction,
regarding factors such as protein adsorption, mass transfer and binding
modules, is required. The conjugation of all these factors will allow the
development of enzymes with enhanced activity towards synthetic materi-
als (Guebitz and Cavaco-Paulo, 2007).
In addition to genetic engineering, reaction engineering (i.e. temperature,
pH, type of agitation and additives) seems to play an important role in the
enzymatic hydrolysis of PA (Silva et al., 2005b, 2007a).
As esterases, amidases are also able to modify PA substrates. Enzymes
and micro-organisms having amidase activity for hydrolyzing polyamides
were patented in 2001 by Crouzet et al. (2001). Recently, an alkali stable
polyamide was isolated from a new strain of Nocardia farcinica and its
potential to hydrolyse water insoluble polyamide oligomers was studied
(Heumann et al., 2009). Another polyamidase from Beauveria brongniartii
was able to hydrolyse polyamide fi bres (Almansa et al., 2008a).
© Woodhead Publishing Limited, 2010