Page 237 - Advances in Textile Biotechnology
P. 237
218 Advances in textile biotechnology
the mild conditions assured by the specificity and selectivity of enzymes.
With reference to silk fibres, the accessibility and reactivity of surface-
available tyrosyl residues still remain to be proved. Overcoming of the
current limitations will allow development of innovative techniques for
surface functionalisation of textile fi bres.
9.6 Other enzymes for protein fi bre functionalisation
In addition to transglutaminases and tyrosinases, other enzymes have
attracted some interest for their ability to interact with protein fi bre sub-
strates. Such is the case for laccases and peroxidases, both belonging to the
group of oxidoreductases. Laccases (p-diphenol : dioxygen oxidoreductase,
EC 1.10.3.2) are Cu-containing enzymes, having four Cu atoms in their
reactive site, which are able to oxidise their substrates by removing one
electron and using oxygen as terminal electron acceptor (Riva, 2006;
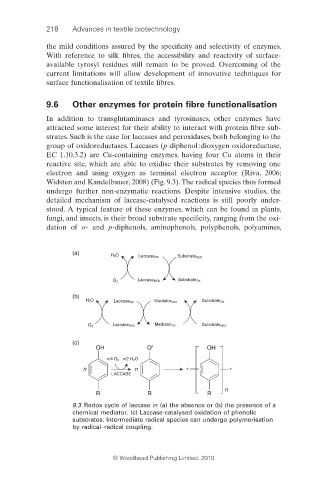
Widsten and Kandelbauer, 2008) (Fig. 9.3). The radical species thus formed
undergo further non-enzymatic reactions. Despite intensive studies, the
detailed mechanism of laccase-catalysed reactions is still poorly under-
stood. A typical feature of these enzymes, which can be found in plants,
fungi, and insects, is their broad substrate specificity, ranging from the oxi-
dation of o- and p-diphenols, aminophenols, polyphenols, polyamines,
(a) H 2 O Laccase OX Substrate RED
O 2 Laccase RED Substrate OX
(b)
H 2 O
Laccase OX Mediator RED Substrate OX
O 2 Laccase RED Mediator OX Substrate RED
(c)
OH O • OH
n/4 O 2 n/2 H 2 O
n n * *
LACCASE
n
R R R
9.3 Redox cycle of laccase in (a) the absence or (b) the presence of a
chemical mediator. (c) Laccase-catalysed oxidation of phenolic
substrates. Intermediate radical species can undergo polymerisation
by radical–radical coupling.
© Woodhead Publishing Limited, 2010