Page 292 - Advances in Textile Biotechnology
P. 292
Enzymatic functionalization of cellulosic fi bres for textiles 273
polysaccharides, which had been first activated by galactose oxidase and
reacted with the thiophilic 4-(4-N-maleimidophenyl)butyric acid hydrazide.
These protein– and enzyme–polysaccharide conjugates were readily
adsorbed onto cotton cellulose powder in aqueous buffer. Most importantly,
these gentle conditions retained the biochemical activities of the antibodies
and enzyme, which were functional on the cellulosic substrate.
11.4.2 Xyloglucan endo-transglycosylase and xyloglucan as
a toolkit for cellulose modifi cation
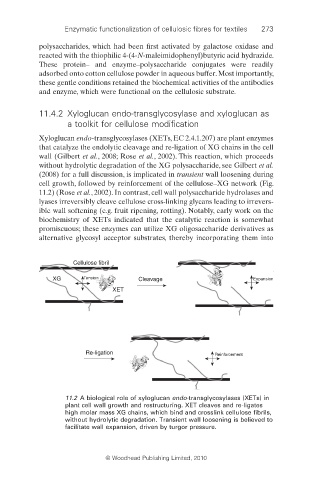
Xyloglucan endo-transglycosylases (XETs, EC 2.4.1.207) are plant enzymes
that catalyze the endolytic cleavage and re-ligation of XG chains in the cell
wall (Gilbert et al., 2008; Rose et al., 2002). This reaction, which proceeds
without hydrolytic degradation of the XG polysaccharide, see Gilbert et al.
(2008) for a full discussion, is implicated in transient wall loosening during
cell growth, followed by reinforcement of the cellulose–XG network (Fig.
11.2) (Rose et al., 2002). In contrast, cell wall polysaccharide hydrolases and
lyases irreversibly cleave cellulose cross-linking glycans leading to irrevers-
ible wall softening (e.g. fruit ripening, rotting). Notably, early work on the
biochemistry of XETs indicated that the catalytic reaction is somewhat
promiscuous; these enzymes can utilize XG oligosaccharide derivatives as
alternative glycosyl acceptor substrates, thereby incorporating them into
Cellulose fibril
XG Tension Cleavage Expansion
XET
Re-ligation Reinforcement
11.2 A biological role of xyloglucan endo-transglycosylases (XETs) in
plant cell wall growth and restructuring. XET cleaves and re-ligates
high molar mass XG chains, which bind and crosslink cellulose fi brils,
without hydrolytic degradation. Transient wall loosening is believed to
facilitate wall expansion, driven by turgor pressure.
© Woodhead Publishing Limited, 2010