Page 132 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 132
106 4 Properties of Aerosol Particles
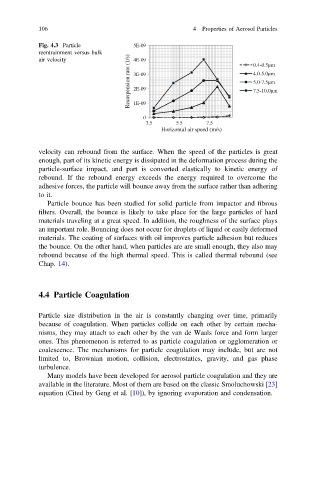
Fig. 4.3 Particle 5E-09
reentrainment versus bulk
Resuspension rate (1/s) 3E-09 4.0-5.0µm
air velocity 4E-09 0.4-0.5µm
5.0-7.5µm
2E-09
7.5-10.0µm
1E-09
0
3.5 5.5 7.5
Horizontal air speed (m/s)
velocity can rebound from the surface. When the speed of the particles is great
enough, part of its kinetic energy is dissipated in the deformation process during the
particle-surface impact, and part is converted elastically to kinetic energy of
rebound. If the rebound energy exceeds the energy required to overcome the
adhesive forces, the particle will bounce away from the surface rather than adhering
to it.
Particle bounce has been studied for solid particle from impactor and fibrous
filters. Overall, the bounce is likely to take place for the large particles of hard
materials traveling at a great speed. In addition, the roughness of the surface plays
an important role. Bouncing does not occur for droplets of liquid or easily deformed
materials. The coating of surfaces with oil improves particle adhesion but reduces
the bounce. On the other hand, when particles are are small enough, they also may
rebound because of the high thermal speed. This is called thermal rebound (see
Chap. 14).
4.4 Particle Coagulation
Particle size distribution in the air is constantly changing over time, primarily
because of coagulation. When particles collide on each other by certain mecha-
nisms, they may attach to each other by the van de Waals force and form larger
ones. This phenomenon is referred to as particle coagulation or agglomeration or
coalescence. The mechanisms for particle coagulation may include, but are not
limited to, Brownian motion, collision, electrostatics, gravity, and gas phase
turbulence.
Many models have been developed for aerosol particle coagulation and they are
available in the literature. Most of them are based on the classic Smoluchowski [23]
equation (Cited by Geng et al. [10]), by ignoring evaporation and condensation.