Page 263 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 263
8.3 Thermochemical Conversion of Fuels 239
Catalytic Catalytic
Ethanol Methanol Gasoline
conversion conversion
Catalytic Catalytic
conversion conversion
Fischer-tropsch
Water-gas
H Syngas
2 shift
Fischer-tropsch
Catalytic
Synthesis Isosynthesis
Alcohols i-C Diesel
4
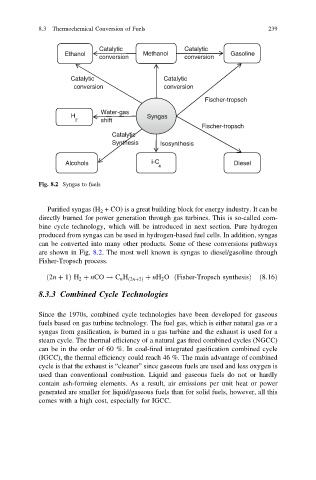
Fig. 8.2 Syngas to fuels
Purified syngas (H 2 + CO) is a great building block for energy industry. It can be
directly burned for power generation through gas turbines. This is so-called com-
bine cycle technology, which will be introduced in next section. Pure hydrogen
produced from syngas can be used in hydrogen-based fuel cells. In addition, syngas
can be converted into many other products. Some of these conversions pathways
are shown in Fig. 8.2. The most well known is syngas to diesel/gasoline through
Fisher-Tropsch process.
ð 2n þ 1Þ H 2 þ nCO ! C n H ð2nþ2Þ þ nH 2 O ð Fisher-Tropsch synthesisÞ ð8:16Þ
8.3.3 Combined Cycle Technologies
Since the 1970s, combined cycle technologies have been developed for gaseous
fuels based on gas turbine technology. The fuel gas, which is either natural gas or a
syngas from gasification, is burned in a gas turbine and the exhaust is used for a
steam cycle. The thermal efficiency of a natural gas fired combined cycles (NGCC)
can be in the order of 60 %. In coal-fired integrated gasification combined cycle
(IGCC), the thermal efficiency could reach 46 %. The main advantage of combined
cycle is that the exhaust is “cleaner” since gaseous fuels are used and less oxygen is
used than conventional combustion. Liquid and gaseous fuels do not or hardly
contain ash-forming elements. As a result, air emissions per unit heat or power
generated are smaller for liquid/gaseous fuels than for solid fuels, however, all this
comes with a high cost, especially for IGCC.