Page 316 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 316
10.3 Flue Gas Desulfurization 293
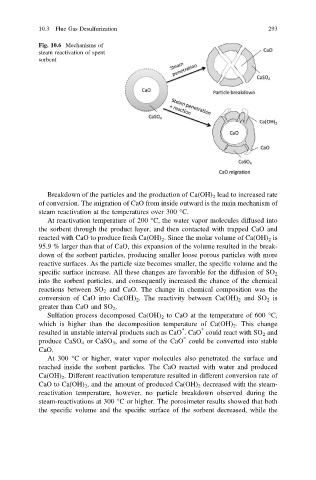
Fig. 10.6 Mechanisms of
steam reactivation of spent
sorbent
Breakdown of the particles and the production of Ca(OH) 2 lead to increased rate
of conversion. The migration of CaO from inside outward is the main mechanism of
steam reactivation at the temperatures over 300 °C.
At reactivation temperature of 200 °C, the water vapor molecules diffused into
the sorbent through the product layer, and then contacted with trapped CaO and
reacted with CaO to produce fresh Ca(OH) 2 . Since the molar volume of Ca(OH) 2 is
95.9 % larger than that of CaO, this expansion of the volume resulted in the break-
down of the sorbent particles, producing smaller loose porous particles with more
reactive surfaces. As the particle size becomes smaller, the specific volume and the
specific surface increase. All these changes are favorable for the diffusion of SO 2
into the sorbent particles, and consequently increased the chance of the chemical
reactions between SO 2 and CaO. The change in chemical composition was the
conversion of CaO into Ca(OH) 2 . The reactivity between Ca(OH) 2 and SO 2 is
greater than CaO and SO 2 .
Sulfation process decomposed Ca(OH) 2 to CaO at the temperature of 600 °C,
which is higher than the decomposition temperature of Ca(OH) 2 . This change
* *
resulted in unstable interval products such as CaO . CaO could react with SO 2 and
*
produce CaSO 4 or CaSO 3 , and some of the CaO could be converted into stable
CaO.
At 300 °C or higher, water vapor molecules also penetrated the surface and
reached inside the sorbent particles. The CaO reacted with water and produced
Ca(OH) 2 . Different reactivation temperature resulted in different conversion rate of
CaO to Ca(OH) 2 , and the amount of produced Ca(OH) 2 decreased with the steam-
reactivation temperature, however, no particle breakdown observed during the
steam-reactivations at 300 °C or higher. The porosimeter results showed that both
the specific volume and the specific surface of the sorbent decreased, while the