Page 62 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 62
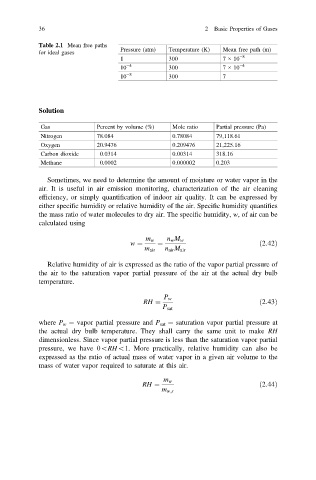
36 2 Basic Properties of Gases
Table 2.1 Mean free paths
Pressure (atm) Temperature (K) Mean free path (m)
for ideal gases
1 300 7 × 10 −8
10 −4 300 7 × 10 −4
10 −8 300 7
Solution
Gas Percent by volume (%) Mole ratio Partial pressure (Pa)
Nitrogen 78.084 0.78084 79,118.61
Oxygen 20.9476 0.209476 21,225.16
Carbon dioxide 0.0314 0.00314 318.16
Methane 0.0002 0.000002 0.203
Sometimes, we need to determine the amount of moisture or water vapor in the
air. It is useful in air emission monitoring, characterization of the air cleaning
efficiency, or simply quantification of indoor air quality. It can be expressed by
either specific humidity or relative humidity of the air. Specific humidity quantifies
the mass ratio of water molecules to dry air. The specific humidity, w, of air can be
calculated using
m w n w M w
w ¼ ¼ ð2:42Þ
m air n air M air
Relative humidity of air is expressed as the ratio of the vapor partial pressure of
the air to the saturation vapor partial pressure of the air at the actual dry bulb
temperature.
P w
RH ¼ ð2:43Þ
P sat
where P w ¼ vapor partial pressure and P sat ¼ saturation vapor partial pressure at
the actual dry bulb temperature. They shall carry the same unit to make RH
dimensionless. Since vapor partial pressure is less than the saturation vapor partial
pressure, we have 0\RH\1. More practically, relative humidity can also be
expressed as the ratio of actual mass of water vapor in a given air volume to the
mass of water vapor required to saturate at this air.
m w
RH ¼ ð2:44Þ
m w;s