Page 64 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 64
38 2 Basic Properties of Gases
Solution
Step 1. Determine the molar weight of the gases
Gas or Vapor Molar weight (g/mol)
H 2 2
H 2 O 18
Air 29
CO 2 44
Step 2. Compute the root-mean-square speed of the gas molecules using Eq. (2.45)
and the kinetic energy of 1 mol of the gas using Eq. (2.46)
Gas or Vapor Molecular weight (g/mol) Equation (2.45) Equation (2.46) (J/mol)
c rms (m/s)
H 2 2 1911.54 3654.0
H 2 O 18 637.18 3654.0
Air 29 501.99 3654.0
CO 2 44 407.54 3654.0
It is apparent that the lighter molecules have the greater root-mean-square speeds
to maintain the equal kinetic energy at the same temperature.
2.1.7 Gas Mean Free Path
The mean free path of a gas is the average distance traveled by gas molecule
between the collisions. Gas mean free path affects the aerosol dynamics and con-
sequently air sampling and cleaning technologies. Gas mean free path may be
estimated from kinetic theory too.
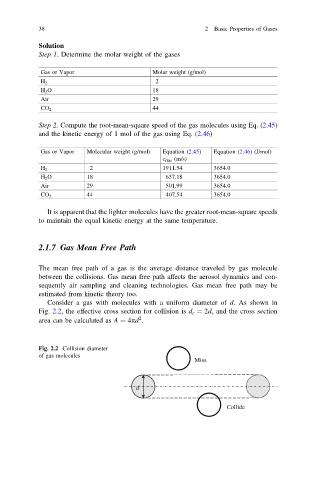
Consider a gas with molecules with a uniform diameter of d. As shown in
Fig. 2.2, the effective cross section for collision is d c ¼ 2d, and the cross section
2
area can be calculated as A ¼ 4pd .
Fig. 2.2 Collision diameter
of gas molecules
Miss
d
Collide