Page 94 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 94
68 3 Basics of Gas Combustion
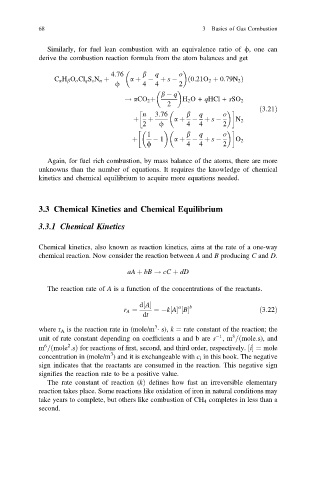
Similarly, for fuel lean combustion with an equivalence ratio of /, one can
derive the combustion reaction formula from the atom balances and get
4:76 b q o
C a H b O o Cl q S s N n þ a þ þ s ð 0:21O 2 þ 0:79N 2 Þ
/ 4 4 2
b q
! aCO 2 þ H 2 O+ qHCl + sSO 2
2
ð3:21Þ
n 3:76 b q o
þ þ a þ þ s N 2
2 / 4 4 2
1 b q o
þ 1 a þ þ s O 2
/ 4 4 2
Again, for fuel rich combustion, by mass balance of the atoms, there are more
unknowns than the number of equations. It requires the knowledge of chemical
kinetics and chemical equilibrium to acquire more equations needed.
3.3 Chemical Kinetics and Chemical Equilibrium
3.3.1 Chemical Kinetics
Chemical kinetics, also known as reaction kinetics, aims at the rate of a one-way
chemical reaction. Now consider the reaction between A and B producing C and D.
aA þ bB ! cC þ dD
The reaction rate of A is a function of the concentrations of the reactants.
d A½ a b
r A ¼ ¼ k½A ½B ð3:22Þ
dt
3
where r A is the reaction rate in (mole/m s), k ¼ rate constant of the reaction; the
3
1
unit of rate constant depending on coefficients a and b are s ,m =ðmole:s), and
2
6
m =ðmole :sÞ for reactions of first, second, and third order, respectively. ½i¼ mole
3
concentration in (mole/m ) and it is exchangeable with c i in this book. The negative
sign indicates that the reactants are consumed in the reaction. This negative sign
signifies the reaction rate to be a positive value.
The rate constant of reaction (kÞ defines how fast an irreversible elementary
reaction takes place. Some reactions like oxidation of iron in natural conditions may
take years to complete, but others like combustion of CH 4 completes in less than a
second.