Page 95 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 95
3.3 Chemical Kinetics and Chemical Equilibrium 69
k is not a real constant, because it is a function of the combustion condition,
especially the temperature. The rate constant of reaction can be determined using a
modified Arrhenius equation
B E A
k ¼ AT exp ð3:23Þ
RT
When B ¼ 0; Eq. (3.23) becomes the Arrhenius equation
E A
k ¼ A exp ð3:24Þ
RT
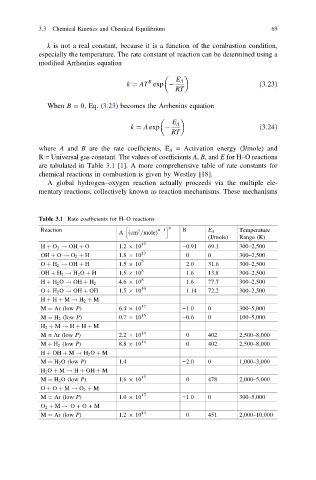
where A and B are the rate coefficients, E A = Activation energy (J/mole) and
R = Universal gas constant. The values of coefficients A, B, and E for H–O reactions
are tabulated in Table 3.1 [1]. A more comprehensive table of rate constants for
chemical reactions in combustion is given by Westley [18].
A global hydrogen–oxygen reaction actually proceeds via the multiple ele-
mentary reactions, collectively known as reaction mechanisms. These mechanisms
Table 3.1 Rate coefficients for H–O reactions
h i n
Reaction 3 n 1 B E A Temperature
A ð cm =moleÞ
(J/mole) Range (K)
H þ O 2 ! OH þ O 1.2 10 17 −0.91 69.1 300–2,500
OH þ O ! O 2 þ H 1.8 10 13 0 0 300–2,500
O þ H 2 ! OH þ H 1.5 10 7 2.0 31.6 300–2,500
OH þ H 2 ! H 2 O þ H 1.5 10 8 1.6 13.8 300–2,500
H þ H 2 O ! OH þ H 2 4.6 10 8 1.6 77.7 300–2,500
O þ H 2 O ! OH þ OH 1.5 10 10 1.14 72.2 300–2,500
H þ H þ M ! H 2 þ M
M ¼ Ar (low P) 6.4 10 17 −1.0 0 300–5,000
M ¼ H 2 (low P) 0.7 10 16 −0.6 0 100–5,000
H 2 þ M ! H þ H þ M
M = Ar (low P) 2.2 10 14 0 402 2,500–8,000
M þ H 2 (low P) 8.8 10 14 0 402 2,500–8,000
H þ OH þ M ! H 2 O þ M
M ¼ H 2 O (low P) 1.4 −2.0 0 1,000–3,000
H 2 O þ M ! H þ OH þ M
M ¼ H 2 O (low P) 1.6 10 17 0 478 2,000–5,000
O þ O þ M ! O 2 þ M
M ¼ Ar (low P) 1.0 10 17 −1.0 0 300–5,000
O 2 +M ! O+O+M
M ¼ Ar (low P) 1.2 10 14 0 451 2,000–10,000