Page 100 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 100
74 3 Basics of Gas Combustion
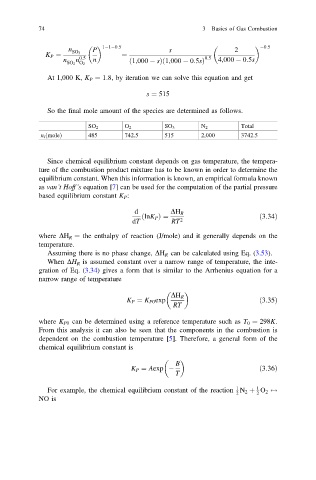
n 1 1 0:5 s 2 0:5
P
SO 3
K P ¼ 0:5 ¼ 0:5
n n n ð1,000 sÞð1,000 0:5sÞ 4,000 0:5s
SO 2 O 2
At 1,000 K, K P ¼ 1:8, by iteration we can solve this equation and get
s ¼ 515
So the final mole amount of the species are determined as follows.
SO 2 O 2 SO 3 N 2 Total
n i ðmoleÞ 485 742.5 515 2,000 3742.5
Since chemical equilibrium constant depends on gas temperature, the tempera-
ture of the combustion product mixture has to be known in order to determine the
equilibrium constant. When this information is known, an empirical formula known
as van’t Hoff’s equation [7] can be used for the computation of the partial pressure
based equilibrium constant K P :
d DH R
ð lnK P Þ ¼ ð3:34Þ
dT RT 2
where DH R ¼ the enthalpy of reaction (J/mole) and it generally depends on the
temperature.
Assuming there is no phase change, DH R can be calculated using Eq. (3.53).
When ΔH R is assumed constant over a narrow range of temperature, the inte-
gration of Eq. (3.34) gives a form that is similar to the Arrhenius equation for a
narrow range of temperature
DH R
K P ¼ K P0 exp ð3:35Þ
RT
where K P0 can be determined using a reference temperature such as T 0 ¼ 298K.
From this analysis it can also be seen that the components in the combustion is
dependent on the combustion temperature [5]. Therefore, a general form of the
chemical equilibrium constant is
B
K P ¼ Aexp ð3:36Þ
T
1 1
For example, the chemical equilibrium constant of the reaction N 2 þ O 2 $
2 2
NO is