Page 101 - Air pollution and greenhouse gases from basic concepts to engineering applications for air emission control
P. 101
3.3 Chemical Kinetics and Chemical Equilibrium 75
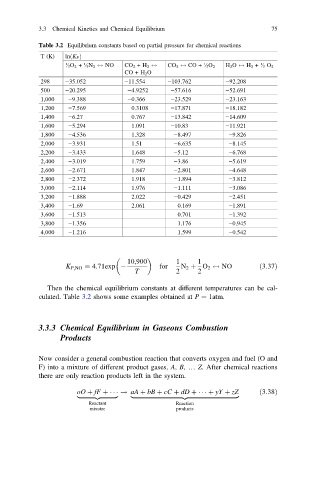
Table 3.2 Equilibrium constants based on partial pressure for chemical reactions
T (K) lnðK P Þ
½O 2 + ½N 2 $ NO CO 2 +H 2 $ CO 2 $ CO + ½O 2 H 2 O $ H 2 + ½ O 2
CO + H 2 O
298 −35.052 −11.554 −103.762 −92.208
500 −20.295 −4.9252 −57.616 −52.691
1,000 −9.388 −0.366 −23.529 −23.163
1,200 −7.569 0.3108 −17.871 −18.182
1,400 −6.27 0.767 −13.842 −14.609
1,600 −5.294 1.091 −10.83 −11.921
1,800 −4.536 1.328 −8.497 −9.826
2,000 −3.931 1.51 −6.635 −8.145
2,200 −3.433 1.648 −5.12 −6.768
2,400 −3.019 1.759 −3.86 −5.619
2,600 −2.671 1.847 −2.801 −4.648
2,800 −2.372 1.918 −1.894 −3.812
3,000 −2.114 1.976 −1.111 −3.086
3,200 −1.888 2.022 −0.429 −2.451
3,400 −1.69 2.061 0.169 −1.891
3,600 −1.513 0.701 −1.392
3,800 −1.356 1.176 −0.945
4,000 −1.216 1.599 −0.542
10,900 1 1
K P;NO ¼ 4:71exp for N 2 þ O 2 $ NO ð3:37Þ
T 2 2
Then the chemical equilibrium constants at different temperatures can be cal-
culated. Table 3.2 shows some examples obtained at P ¼ 1atm.
3.3.3 Chemical Equilibrium in Gaseous Combustion
Products
Now consider a general combustion reaction that converts oxygen and fuel (O and
F) into a mixture of different product gases, A, B, … Z. After chemical reactions
there are only reaction products left in the system.
oO þ fF þ ! aA þ bB þ cC þ dD þ þ yY þ zZ ð3:38Þ
|fflfflfflfflfflfflfflfflfflffl{zfflfflfflfflfflfflfflfflfflffl} |fflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflffl{zfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflfflffl}
Reactant Reaction
mixutre products