Page 151 - An Introduction to Analytical Atomic Spectrometry - L. Ebdon
P. 151
Page 135
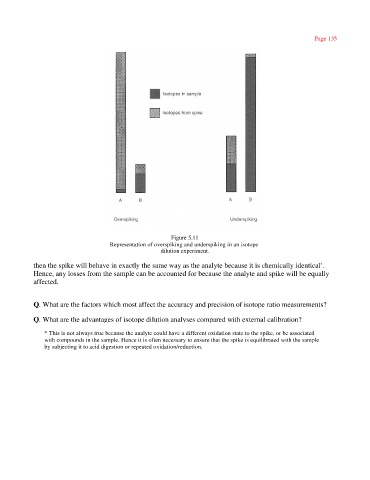
Figure 5.11
Representation of overspiking and underspiking in an isotope
dilution experiment.
then the spike will behave in exactly the same way as the analyte because it is chemically identical .
*
Hence, any losses from the sample can be accounted for because the analyte and spike will be equally
affected.
Q. What are the factors which most affect the accuracy and precision of isotope ratio measurements?
Q. What are the advantages of isotope dilution analyses compared with external calibration?
* This is not always true because the analyte could have a different oxidation state to the spike, or be associated
with compounds in the sample. Hence it is often necessary to ensure that the spike is equilibrated with the sample
by subjecting it to acid digestion or repeated oxidation/reduction.