Page 24 - An Introduction to Analytical Atomic Spectrometry - L. Ebdon
P. 24
Page 9
calibration curve, i.e. it must never be more concentrated than the strongest standard and preferably not
more dilute than the weakest.
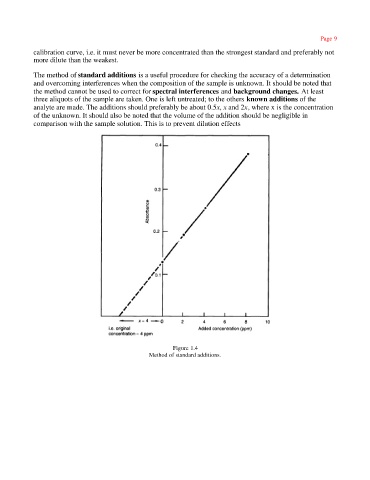
The method of standard additions is a useful procedure for checking the accuracy of a determination
and overcoming interferences when the composition of the sample is unknown. It should be noted that
the method cannot be used to correct for spectral interferences and background changes. At least
three aliquots of the sample are taken. One is left untreated; to the others known additions of the
analyte are made. The additions should preferably be about 0.5x, x and 2x, where x is the concentration
of the unknown. It should also be noted that the volume of the addition should be negligible in
comparison with the sample solution. This is to prevent dilution effects
Figure 1.4
Method of standard additions.