Page 42 - An Introduction to Analytical Atomic Spectrometry - L. Ebdon
P. 42
Page 27
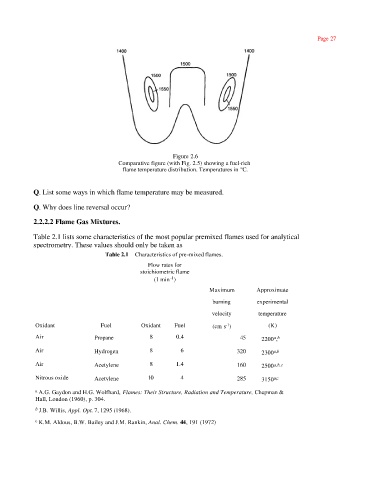
Figure 2.6
Comparative figure (with Fig. 2.5) showing a fuel-rich
flame temperature distribution. Temperatures in °C.
Q. List some ways in which flame temperature may be measured.
Q. Why does line reversal occur?
2.2.2.2 Flame Gas Mixtures.
Table 2.1 lists some characteristics of the most popular premixed flames used for analytical
spectrometry. These values should only be taken as
Table 2.1 Characteristics of pre-mixed flames.
Flow rates for
stoichiometric flame
(1 min )
-1
Maximum Approximate
burning experimental
velocity temperature
Oxidant Fuel Oxidant Fuel (cm s ) (K)
-1
Air Propane 8 0.4 45 2200 ,
a b
Air Hydrogen 8 6 320 2300 a,b
Air Acetylene 8 1.4 160 2500 a,b,c
Nitrous oxide Acetylene 10 4 285 3150 a,c
a A.G. Gaydon and H.G. Wolfhard, Flames: Their Structure, Radiation and Temperature, Chapman &
Hall, London (1960), p. 304.
b J.B. Willis, Appl. Opt. 7, 1295 (1968).
c K.M. Aldous, B.W. Bailey and J.M. Rankin, Anal. Chem. 44, 191 (1972)