Page 114 - Applied Process Design For Chemical And Petrochemical Plants Volume II
P. 114
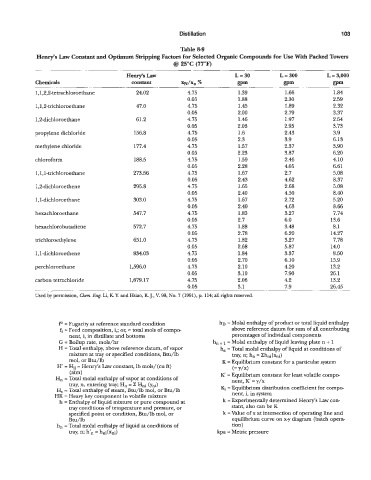
Distillation 1 03
Table 8-9
Henry's Law Constant and Optimum Stripping Factors for Selected Organic Compounds for Use With Packed Towers
@I 25°C (77°F)
- .. .- .....
Henry's Law L = 30 L = 300 L = 3,000
Chemicals constant xN/Xo % am gPm wm
... .. .- ~. . .~ ~
1,1,2,2-tetrachloroethane 24.02 4.75 1.39 1.66 1.84
0.05 1.88 2.30 2.59
1,1,2-trichloroethane 47.0 4.75 1.45 1.89 2.32
0.05 2.00 2.79 3.37
1,2-dichloroethane 61.2 4.75 1.46 1.97 2.54
0.05 2.03 2.95 3.73
propylene dichloride 136.8 4.75 1.6 2.43 3.9
0.05 2.3 3.9 6.13
methylene chloride 177.4 4.75 1.57 2.37 3.90
0.05 2.23 3.87 6.20
chloroform 188.5 4.73 1.59 2.46 4.10
0.05 2.28 4.05 6.61
1 ,l,l-trichloroethane 273.56 4.75 1.67 2.7 5.08
0.03 2.43 4.62 8.37
i ,2-dichloroethene 295.8 4.75 1.65 2.68 3.08
0.05 2.40 4.30 8.40
1 ,I-dichloroethane 303.0 4.75 1.67 2.72 5.20
0.05 2.40 4.63 8.66
hexachloroethane 547.7 4.75 1.83 3.27 7.74
0.05 2.7 6.0 13.6
hexachlorobutadiene 572.7 4.75 1.88 3.48 8.1
0.03 2.78 6.20 14.27
trichloroethylene 651.0 4.75 1.82 3.27 7.78
0.05 2.68 5.87 14.0
1 ,I-dichloroethene 834.03 4.73 1.84 3.37 8.50
0.05 2.70 6.10 13.9
perchloroethane 1,396.0 4.75 2.10 4.20 13.2
0.05 3.10 7.90 26.1
carbon tetrzchloride 1,679.17 4.75 2.06 4.2 13.2
0.05 3.1 7.9 26.43
. .- ..... ._ ~-
Used by permission, Chem. Eng. Li, K Y. and Hsiao, K. J., Lr. 98, No. 7 (1991), p. 114; all rights reserved.
f" = Fugacity at reference standard condition hD = Molal enthalpy of product or total liquid enthalpy
fi = Feed composition, i,; or, = total mols of compo- above reference datum for sum of all contributing
nent, i, in distillate and bottoms percentages of individual components
G = Boilup rate, mols/hr h, + 1 = Molal enthalpy of liquid leaving plate n + 1
H = Total enthalpy, above reference datum, of vapor ha = Total molal enthalpy of liquid at conditions of
mixture at tray or specified conditions, Btu/lb tray, n; h, = Zh,i(x,i)
mol, or Btu/lb K = Equilibrium constant for a particular system
H' = Hij = Henry's Law constant, lb mols/(cu ft) (= Y/X)
(at4 K' = Equilibrium constant for least volatile compe
H, = Total molal enthalpy of vapor at conditions of nent, K' = y/x
tray, n, entering tray; H, = 2 H,i (y,i)
H, = Total enthalpy of steam, Btu/lb mol, or Btu/lb Ki = Equilibrium distribution coefficient for compe
HK = Heavy key component in volatile mixture nent, i, in system
h = Enthalpy of liquid mixture or pure compound at k = Experimentally determined Henry's Law con-
tray conditions of temperature and pressure, or stant, also can be K
specified point or condition, Btu/lb mol, or k = Value of x at intersection of operating line and
Btu/lb equilibrium curve on x-y diagram (batch opera-
h, = Total molal enthalpy of liquid at conditions of tion)
tray, n; h', = h,i(x,i) kpa = Metric pressure