Page 234 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 234
sterilised containers and aseptic
processing. Consider a terminal
microbial reduction process
Use sterile filtration, pre-
Yes
containers and aseptic
Use sterile filtration,
pre-sterilised
No
processing
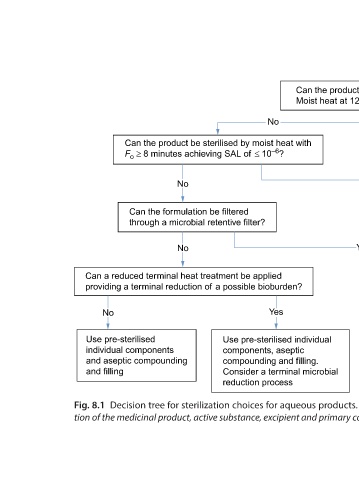
Can the product be sterilised by Moist heat at 121°C for 15 min? Yes Use autoclaving at 121°C for 15 min Yes Use moist heat with F o ³ 8 min Yes Can a reduced terminal heat treatment be applied providing a terminal reduction of a possible bioburden? Developing new products Decision tree for sterilization choices fo
Can the product be sterilised by moist heat with F o ³ 8 minutes achieving SAL of £ 10 –6 ? No Can the formulation be filtered through a microbial retentive filter? No Can a reduced terminal heat treatment be applied providing a terminal reduction of a possible bioburden?
No Yes Use pre-sterilised individual components, aseptic compounding and filling. Consider a terminal microbial reduction process tion of the medicinal product, active substance, excipient and primary container, April 2016, European Medicines Agency.
No Use pre-sterilised individual components and aseptic compounding and filling Fig. 8.1