Page 236 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 236
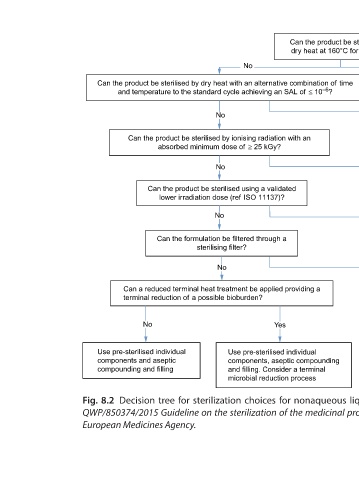
temperature to the standard cycle achieving an SAL of £10 –6
Can a reduced terminal heat treatment be applied providing a
Use dry heat with alternative combination of time and
Use sterilisation with an absorbed
Use sterilisation by validated
minimum dose of ³ 25 kGy
³ 160°C for ³ 120 min
terminal reduction of a possible bioburden?
Use sterilisation at
irradiation dose
Yes
Yes
Yes
Can the product be sterilised by 214 Yes Assurance of sterility for sensitive combination products and materials Yes No Use sterile filtration, pre-sterilised Use sterile filtration, pre- containers and aseptic processing. sterilised containers and aseptic processing Consider a terminal microbial reduction process Decision tre
dry heat at 160°C for 120 min?
Yes
Can the product be sterilised by dry heat with an alternative combination of time
and temperature to the standard cycle achieving an SAL of £ 10 –6 ?
No Yes Use pre-sterilised individual components, aseptic compounding and filling. Consider a terminal microbial reduction process
No Can the product be sterilised by ionising radiation with an absorbed minimum dose of ³ 25 kGy? No Can the product be sterilised using a validated lower irradiation dose (ref ISO 11137)? No Can the formulation be filtered through a sterilising filter? No Can a reduced terminal heat treatment be applied providing