Page 97 - Assurance of Sterility for Sensitive Combination Products and Materials
P. 97
84
impermeable
material may
(max temp)
√ (160°C)
Dry Heat
be used
No
No
no
or Beta radiation
Gamma/E-Beam
material may be
impermeable
used
No
√
√
√
materials are
incompatible
fibre based
Peroxide
(Plasma)
natural
No
No
√
√
at least a part of
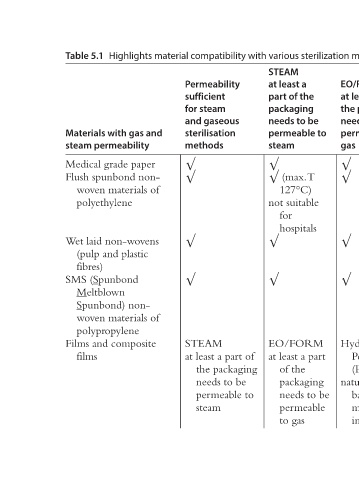
Highlights material compatibility with various sterilization methods [27].
the packaging
permeable to
needs to be
EO/FORM
gas
√
√
√
√
permeable to
not suitable
needs to be
√ (max. T
hospitals
packaging
part of the
127°C)
at least a
STEAM Hydrogen Assurance of sterility for sensitive combination products and materials Dry Heat Gamma/E- Hydrogen EO/FORM (max temp) Beam Peroxide at least a part impermeable or Beta (Plasma) of the material may radiation natural fibre packaging be used impermeable based needs to be material ma
for
steam
√
√
√
Permeability sufficient for steam and gaseous sterilisation methods √ √ √ √ STEAM at least a part of the packaging needs to be permeable to steam
Materials with gas and steam permeability Medical grade paper Flush spunbond non- woven materials of polyethylene Wet laid non-wovens (pulp and plastic SMS (Spunbond Meltblown Spunbond) non- woven materials of polypropylene Films and composite
Table 5.1 fibres) films