Page 136 - Basic physical chemistry for the atmospheric sciences
P. 136
1 2 2 Basic physical chemistry
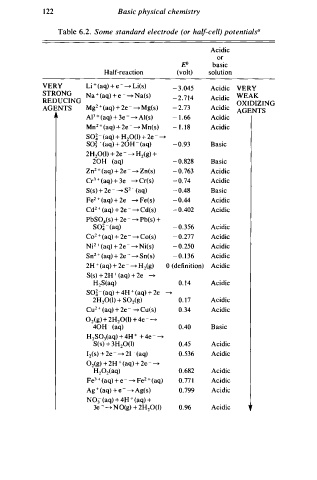
Table 6.2. Some standard electrode (or half-cell) potentialsa
Acidic
or
g.i basic
Half-reaction (volt) solution
e
VERY Li ( aq) + - - Li(s) - 3 .045 Acidic VERY
+
STRONG Na ( aq) + e - - Na(s) WEAK
+
REDUCING 2 - 2 .714 Acidic OXIDIZING
AGENTS Mg + (aq) + 2e - - Mg(s) - 2 .73 Acidic AGENTS
AP + (aq) + 3e - - Al(s) - 1 .66 Acidic
Mn2 + (aq) + 2e - - Mn(s) - 1 . 1 8 Acidic
SO� ( aq) + H 0(1) + 2e - -
-
2
so� - ( aq + ) 2 oH - ( aq) - 0 .93 Basic
2H20(1) + 2e - - Hz(g) +
20H (aq) - 0 . 8 28 Basic
Zn2 + (aq) + 2e - - Z n(s) - 0 .763 Acidic
Cr3 + (aq) + 3e - - Cr(s) - 0 . 7 4 Acidic
S(s) + 2e - - s2 - (aq) - 0 .48 Basic
Fe 2 + ( aq) + 2e - - Fe(s) - 0 .44 Acidic
Cd2 + (aq) + 2e - - Cd(s) - 0 .402 Acidic
PbS04(s) + 2e - - Pb(s) +
so� - (aq) - 0 . 3 56 Acidic
2
Co + (aq) + 2e - - Co(s) - 0 .277 Acidic
Ni2 + (aq) + 2e - - Ni(s) - 0 . 2 50 Acidic
Sn2 + (aq) + 2e - - Sn(s) - 0 . 1 3 6 Acidic
2H + (aq) + 2e - - H (g) 0 (definition) Acidic
2
S(s) + 2H + (aq) + 2e - -
H2S(aq) 0. 1 4 Acidic
+
SQ� - (aq) 4 H + ( aq) + 2 e - -
2H20(1) + S02(g) 0. 1 7 Acidic
2
Cu + ( aq) + 2e - - c u(s) 0.34 Acidic
02(g) + 2H20(1) + 4e - -
40H - (aq) 0.40 Basic
H2S03(aq) + 4H + + 4e - -
S(s) + 3H20(1) 0.45 Acidic
I (s) + 2e - - 2 I - (aq) 0. 536 Acidic
2
02(g) + 2H + (aq) + 2e - -
H 0z(aq) 0.682 Acidic
2
Fe3 + ( aq) e - - Fe2 + (aq) 0.77 1 Acidic
+
Ag + ( aq) + e - - A g(s) 0.799 Acidic
+
N03 (aq) 4 H + (aq) +
3e - - N O(g) + 2H20(1) 0.96 Acidic