Page 137 - Basic physical chemistry for the atmospheric sciences
P. 137
Oxidation-reduction reactions 1 2 3
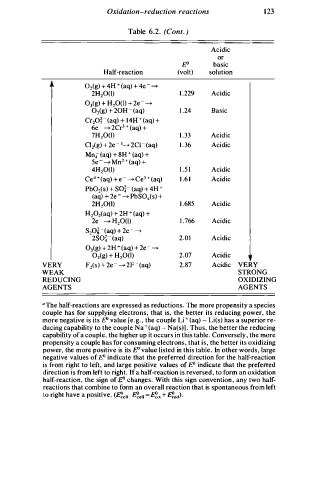
Table 6.2. (Cont. )
Acidic
or
E" basic
Half-reaction (volt) solution
Oz(g) 4 H + ( aq) 4 e - --'>
+
+
2H20(I) 1 . 229 Acidic
03(g) + H20(1) + 2e - --'>
02(g) + 20H - ( aq) 1 . 24 Basic
Cr2o� - (aq) + 14H + (aq) +
6e - -'> 2Cr 3 + (aq) +
7H20(I) 1 . 33 Acidic
Cl2(g) + 2e - 1--'> 2CI - (aq) 1 . 3 6 Acidic
Mn.;- (aq) + 8H + (aq) +
se - --'> Mn2 + (aq) +
4H20(I) 1 . 5 1 Acidic
Ce4 + (aq) + e - --'> Ce 3 + (aq) 1 . 6 1 Acidic
-
Pb02(s) + SO� ( aq) + 4H +
(aq) + 2e - --'> PbS04(S) +
2H20(1) 1 . 685 Acidic
H20z(aq) + 2H + ( aq) +
2e - --'> H20(1) 1 . 766 Acidic
-
S20� ( aq) + 2e - --'>
2so� - ( aq) 2.01 Acidic
0 (g) + 2H + (aq) + 2e - --'>
3
02(g) + H20(1) 2.07 Acidic
V E RY F2(s) + 2e - --'> 2F - (aq) 2.87 Acidic VERY
WEAK STRONG
REDUCING OXIDIZING
AGENTS AGENTS
0 T he half-reactions are expressed as reductions. The more propensity a species
couple has for supplying electrons, that is, the better its reducing power, the
more negative is its E" value [e .g. , the couple Li + (aq) - Li(s) has a superior re
ducing capability to the couple Na ' (aq) - Na(s)] . Thus, the better the reducing
e
capability of a couple, the higher up it occurs in this tabl . Conversely, the more
s
propensity a couple has for consuming electron , that is, the better its oxidizing
power, the more positive is its E" value listed in this table . In other words, large
negative values of E" indicate that the preferred direction for the half-reaction
is from right to left, and large positive values of E" indicate that the preferred
direction is from left to right. If a half-reaction is reversed , to form an oxidation
half-reaction, the sign of E" changes. With this sign convention , any two half
reactions that combine to form an overall reaction that is spontaneous from left
to right have a positive. (�ell �ell = f!x + �ed).