Page 145 - Basic physical chemistry for the atmospheric sciences
P. 145
Oxidation-reduction reactions I . I I
1 . 0 Hzo- - - � h
' ""I. < <
' o ·
0.8 Oxidizin g , O s; 9 q
P
acidic
0.6 Oxidizin g , '
basic
� 0.4 pH = 4.0
0
>
:;- 0.2
LI.l pH = 9.0
0.0 - , !f20
Reducing,
H; ' ' , , acidic
-0.2
l?, Reducin g ,
basic
-0.4 � .,.
"'l!o s;
9 /J/ 1 '
-0.6
0 2 4 6 8 1 0 1 2 1 4
pH
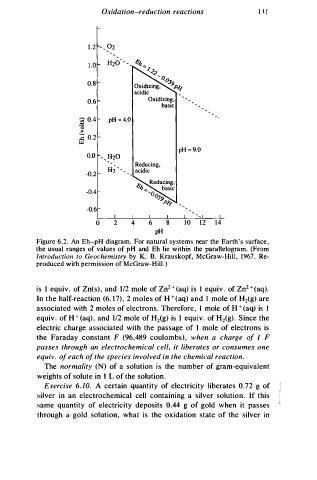
Figure 6.2. An Eh-pH diagram. For natural systems near the Earth's surface,
the usual ranges of values of pH and Eh lie within the parallelogram. (From
Introduction to Geochemistry by K. B. Krauskopf, McGraw-Hill, 1 9 67. Re
produced with permission of McGraw-Hill . )
2
is I equiv . of Zn(s), and 1 / 2 mole of Zn + ( aq) is I equiv . of Zn2 + (aq).
In the half-reaction (6. 1 7), 2 moles of H + (aq) and I mole of H2(g) are
associated with 2 moles of electrons. Therefore, I mole of H ( aq) is I
+
)
equiv. of H + ( aq , and 1 / 2 mole of H2(g) is 1 equiv. of H2(g). Since the
electric charge associated with the passage of I mole of electrons is
the Faraday constant F (96,489 coulombs), when a charge o f 1 F
passes through an electrochemical cell, it liberates or consumes one
equiv. o f each o f the species involved in the chemical reaction.
The normality (N) of a solution i s the number of gram-equivalent
weights of solute in I L of the solution.
Exercise 6. /0. A certain quantity of electricity liberates 0.72 g of
silver in an electrochemical cell containing a silver solution. If this
same quantity of electricity deposits 0.44 g of gold when it passes
through a gold solution, what is the oxidation state of the silver in