Page 152 - Basic physical chemistry for the atmospheric sciences
P. 152
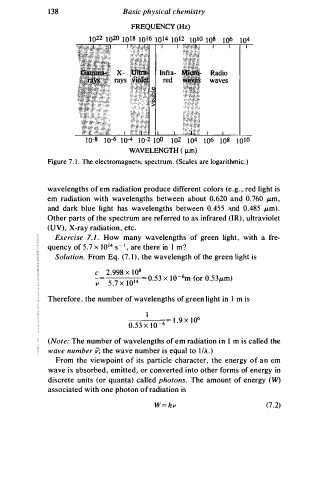
138 Basic physical chemistry
FREQUENCY (Hz)
1022 1 0 20 10 1s 1 0 1 6 10 14 1012 1 0 10 1 0 s 106 104
Radio
waves
1 0 -s 1 0 -6 1 0 -4 1 0 -2 t o<> 102 1 04 106 10s 1 010
W A VELENGTH ( µm)
Figure 7.1. The electromagnetu; spectrum. (Scales are logarithmic.)
wavelengths of em radiation produce different colors (e.g. , red light is
em radiation with wavelengths between about 0.620 and 0.760 µ.m,
and dark blue light has wavelengths between 0.455 ::tnd 0.485 µ.m).
Other parts of the spectrum are referred to as infrared (IR), ultraviolet
(UV), X-ray radiation, etc.
Exercise 7.1 . How many wavelengths of green light, with a fre
5
quency of . 7 x 1 0 1 4 s - 1 , are there in I m ?
Solution. From Eq. (7. 1 ) , the wavelength of the green light is
c 2 . 9 98 x 1 0 8
1 4 0.53 x 1 0 - 6 m (or 0.53µ.m)
_
� 5 7 1 0
x
Therefore, the number of wavelengths of green light in I m is
I 1 . 9 1 0 6
0.53 x 1 0 - 6 x
(Note: The number of wavelengths of m e radiation n I m i s called the
i
wave number ii; the wave number is equal to I / A . )
From the viewpoint f its particle character, the energy o f a n em
o
i
wave is absorbed, emitted, or converted n to other forms of energy in
discrete units (or quanta) called photons. The amount of energy ( W)
associated with one photon f radiation is
o
W = h v (7. 2 )