Page 192 - Basic physical chemistry for the atmospheric sciences
P. 192
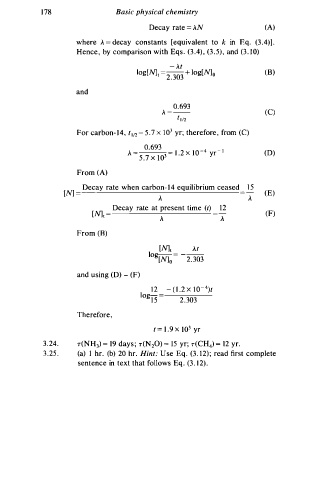
1 78 Basic physical chemistry
Decay rate = AN (A)
where A = decay constants [equivalent to k in Eq. (3 .4)] .
Hence, by comparison with Eqs. (3 .4) , (3 . 5), and (3. 1 0)
- At
. 3 (B)
log [N]r = 2 03 + log[N] o
and
0.693
(C)
A = -
t 1/2
For carbon- 1 4 , t 1 12 = 5 . 7 x 1 0 3 yr; therefore, from (C)
_ 0 .693 l 3 _ - _4 _ I
x
A l . 2 x l 0 yr (D)
- . 7
5 0
From (A)
Decay rate when carbon- 1 4 equilibrium ceased 1 5
[N] = (E)
A A
Decay rate at present time (!) 1 2
1
[N1 = A = A (F)
From (B)
[N] t At
log = -
[N] 0 2. 303
and using (D) - (F)
1 2 - ( l . 2 x 1 0 - 4 )1
log15 =
2 . 3 03
Therefore,
t = 1 . 9 x 1 0 3 yr
3 . 2 4. T(NH ) = 19 days ; T(N20) = 15 yr; T(CH4) = 12 yr.
3
U
3
3 . 25 . (a) I hr. (b) 20 hr. Hint: s e Eq. ( . 1 2) ; read first complete
sentence in text that follows Eq . (3 . 1 2 ) .