Page 43 - Basic physical chemistry for the atmospheric sciences
P. 43
Chemical thermodynamics 29
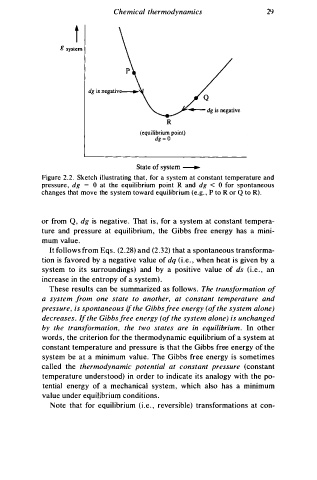
t
g system
R
(equilibrium point)
dg = O
State of system -----.
Figure 2.2. Sketch illustrating that , for a system at constant temperature and
e = 0 at the equilibrium point R and dg < 0 for spontaneous
pressur , dg
changes that move the system toward equilibrium (e.g. , P to R or Q to R) .
e
or from Q , dg is negativ . That is, for a system at constant tempera
ture and pressure at equilibrium, the Gibbs free energy has a mini
mum value.
It follows from E q s . (2.28) and (2. 3 2 ) that a spontaneous transforma
i
tion is favored by a negative value of dq ( i . e . , when heat s given by a
system to its surroundings) and by a positive value of ds (i.e. , an
increase in the entropy of a system).
These results can be summarized as follows. The transformation o f
a system f r om one state to another, at constant temperature and
pressure, is spontaneous if the Gibbs f r ee energy (of the system alone)
decreases. If the Gibbs f r ee energy (of the system alone) is unchanged
by the transformation, the two states are in equilibriu . In other
m
wor s , the criterion for the thermodynamic equilibrium of a system at
d
constant temperature and pressure is that the Gibbs free energy of the
system be at a minimum value. The Gibbs free energy is sometimes
called the thermodynamic potential at constant pressure (constant
temperature understood) in order to indicate its analogy with the po
tential energy of a mechanical system, which also has a minimum
value under e q uilibrium conditions.
Note that for equilibrium (i. . , reversible) transformations at con-
e