Page 47 - Basic physical chemistry for the atmospheric sciences
P. 47
Chemical thermodynamics :n
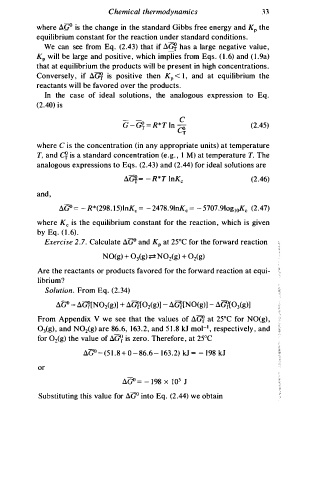
where A.GO is the change in the standard Gibbs free energy and KP the
equilibrium constant for the reaction under standard conditions.
We can see from Eq. (2.43) that if A.Gf has a large negative value,
(
s
KP will be large and positive, which implies from Eq . ( l .6) and l . 9a)
that at equilibrium the products will be present in high concentrations.
Conversely, if A."Gf is positive then K < , and at equilibrium the
l
P
reactants will be favored over the products.
In the case of ideal solutions, the analogous expression to Eq.
(2.40) is
- - c
G - Gt = R * T ln Of (2.45)
i
where C s the concentration (in any appropriate units) at temperature
T, and C¥ is a standard concentration (e. g . , l M) at temperature T. The
analogous expressions to Eqs. (2.43) and (2.44) for ideal solutions are
(2.46)
and,
AGO = - R * ( 298. 5 )lnKc = - 2 478.9lnKc = - 5 707.91og1oKc (2.47)
l
where Kc is the equilibrium constant for the reaction, which is given
by Eq. ( l . 6 ) .
Exercise . 7 . Calculate AGO and KP at 25°C for the forward reaction
2
N O(g) + 0 (g) +:± N Oi(g) + Oi(g)
3
Are the reactants or products favored for the forward reaction at equi
librium?
Solution. From Eq. (2.34)
A GO = A(;?[N02(g)] + A.(;?[Oi(g)] - AG?[NO(g)] - AG?[Oig)]
)
From Appendix V we see that the values of AG/ at 25°C for NO(g ,
1
03(g), and N02(g) are 86.6, 1 6 3 . 2 , and 1 . 8 kJ mo1- , respectively, and
5
for Oi(g) the value of A.G/ is zero. Therefore, at 25°C
AG -.0 = ( 5 1 . 8 + 0 - 8 6.6 - 1 6 3 . 2 ) kJ = - 1 98 kJ
or
A.GO = - 1 98 x 1 0 3 J
Substituting this value for AGO into Eq. (2.44) we obtain