Page 50 - Basic physical chemistry for the atmospheric sciences
P. 50
36 Basic physical chemistry
where e and T are the vapor pressure and temperature of the system,
e
respectively, and e s is the saturation vapor pressur . For a droplet of
radius R, Eq. (2.53) becomes
ilE = 47TR u - n�1TR kT In (;) (2.54)
3
2
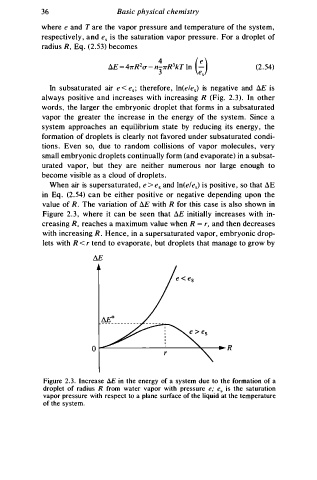
In subsaturated air e < e.; therefore, ln(ele.) is negative and 6.E is
always positive and increases with increasing R (Fig. 2 . 3 ) . In other
wor s , the larger the embryonic droplet that forms in a subsaturated
d
vapor the greater the increase in the energy of the syste . Since a
m
s y stem approaches an equilibrium state by reducing its energy, the
formation of droplets is clearly not favored under subsaturated condi
tions. Even so, due to random collisions of vapor molecules, very
small embryonic droplets continually form (and evaporate) in a subsat
urated vapor, but they are neither numerous nor large enough to
become visible as a cloud of droplets.
When air is supersaturated, e e . and ln(ele.) s positive, so that .:lE
i
>
in Eq. (2.54) can be either positive or negative depending upon the
value of R. The variation of .:lE with R for this case is also shown in
Figure 2 . 3 , where it can be seen that ilE initially increases with in
=
creasing R, reaches a maximum value when R r, and then decreases
with increasing R. Hence, in a supersaturated vapor, embryonic drop
lets with R < r tend to evaporate, but droplets that manage to grow by
Figure 2.3. Increase t:,.E in the energy of a system due to the formation of a
droplet of radius R from water vapor with pressure e; e. is the saturation
vapor pressure with respect to a plane surface of the liquid at the temperature
of the system.