Page 79 - Basic physical chemistry for the atmospheric sciences
P. 79
Chemical kinetics ""
(b) What is the relationship between k and the rate cocfli
cients for the reaction mechanism chosen in question (a) ' !
(c) What is the relationship between the equilibrium con
stant (Kc) for the overall reaction and the rate coeffi
cients for the reaction mechanism chosen in question (a)?
fo
3 . 1 6 . Calculate the approximate maximum rate r a gaseous bi
molecular chemical reaction at 0.50 atm and - 20°C, given
0
3
that the molecular diameter (s) is . 0 x 1 0 - 1 m and the aver
8
age speed of a molecule (c) is 4 1 m s - 1 ? How long would it
take to consume the reacting gases at this rate? (Compare the
answers with the solutions to Exercise . 5 . )
3
3 . 1 7 . B y inspecting and generalizing Figure . 2 , show that
3
!!>.Hrx = Ea (forward) - Ea (reverse)
Hence, show that for an endothermic reaction, the activa
l
tion energy in the forward direction wi l , in general, exceed
the heat of reaction.
3 . 1 8 . Many reaction rates roughly double when the temperature
)
increases by about l 0 °C above room temperature (20°C .
What i s the activation energy of a reaction that obeys this
rule exactly?
fo
6
3. 1 9 . The activation energy r a reaction is 5 k J mo - 1 • What s
1
i
o
t
o
the effect n t h e rate of the reaction f changing h e temper
ature from 25°C to 32°C?
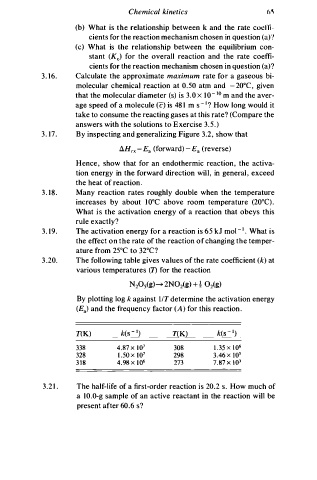
3 . 20. The following table gives values of the rate coefficient (k) at
various temperatures (1) for the reaction
By plotting log k against l/T determine the activation energy
(Ea) and the frequency factor (A) for this reaction.
T(K) T(K)
338 4.87 x 1 0 7 308 1 . 35 x 1 0 6
328 1 . 50 x 1 0 7 298 3 . 46 X 105
3 1 8 4.98 x 1 0 6 273 7.87 x 1 0 3
s
3 . 2 l . The half-life of a first-order reaction is 20.2 . How much of
a 10.0-g sample of an active reactant in the reaction will be
present after 60.6 s?