Page 76 - Basic physical chemistry for the atmospheric sciences
P. 76
62 Basic physical chemistry
(f) A catalyst for a forward reaction has an equal effect on
the reverse reaction.
(g) Gases that have small residence times in the atmosphere
tend to have large spatial variabilities.
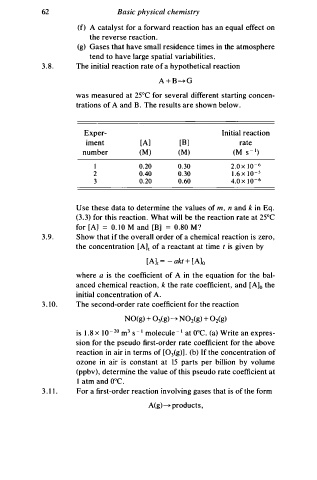
3 . 8 . The initial reaction rate f a hypothetical reaction
o
A + B � G
was measured at 25°C for several different starting concen
B
trations of A and . The results are shown below.
Ex per- Initial reaction
iment [A] [B] rate
-
number (M) (M) (M s 1 )
I 0 .20 0.30 2.0 x 1 0 - 6
2 0.40 0 . 3 0 1 . 6 x 1 0 - 5
3 0.20 0.60 4.0 x 1 0 - 6
Use these data to determine the values of , n and i n Eq.
m
k
(3.3) for this reaction. What will be the reaction rate at 25°C
for [A] = 0. 1 0 M and [BJ = 0 . 8 0 M ?
3 . 9 . S h ow that f the overall order of a chemical reaction is zero,
i
the concentration [Alt of a reactant at time t is given by
[Alt = - akt + [A]0
where a is the coefficient of A in the equation for the bal
anced chemical reactio , k the rate coefficient, and [A]0 the
n
initial concentration of A.
fo
3 . 1 0 . The second-order rate coefficient r the reaction
NO(g) + Oig)� N02(g) + Oz{g)
3
is l . 8 x 1 0 - 2 0 m s - 1 molecule - 1 at 0°C. (a) Write an expres
sion for the pseudo first-order rate coefficient for the above
reaction in air in terms of [03(g)] . (b) If the concentration of
ozone in air is constant at 15 parts per billion by volume
(ppbv), determine the value of this pseudo rate coefficient at
I atm and 0°C.
3 . 1 l . For a first-order reaction involving gases that is of the form
A(g)� products,