Page 73 - Basic physical chemistry for the atmospheric sciences
P. 73
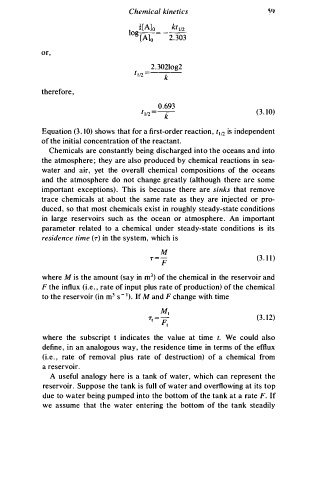
Chemical kinetics
or,
2 . 3 02log2
t 12 =
1
k
therefore,
0 . 6 93
t 112 = -- (3. 10)
k
Equation (3. 1 0) shows that for a first-order reaction, t 112 is independent
of the initial concentration of the reactant.
Chemicals are constantly being discharged into the oceans and into
the atmosphere; they are also produced by chemical reactions in sea
water and air, yet the overall chemical compositions of the oceans
and the atmosphere do not change greatly (although there are some
important exceptions). This is because there are sinks that remove
trace chemicals at about the same rate as they are injected or pro
duced, so that most chemicals exist in roughly steady-state conditions
in large reservoirs such as the ocean or atmosphere. An important
parameter related to a chemical under steady-state conditions is its
residence time (r) in the system, which is
M
r = - (3. 1 1 )
F
3
where M i s the amount (say in m ) of the chemical in the reservoir and
F the influx (i.e. , rate of input plus rate of production) of the chemical
to the reservoir (in m3 s- 1). If M a nd F change with time
M,
T1 = p (3 . 1 2 )
I
where the subscript t indicates the value at time t. We could also
define, in an analogous way, the residence time in terms of the efflux
(i. . , rate of removal plus rate of destruction) of a chemical from
e
a reservoir.
A useful analogy here is a tank of water, which can represent the
reservoir. Suppose the tank is full of water and overflowing at its top
due to water being pumped into the bottom of the tank at a rate F. If
we assume that the water entering the bottom of the tank steadily