Page 69 - Basic physical chemistry for the atmospheric sciences
P. 69
Chemical kinetics
Activated complex
. . �
H . . . . .
i
f+ . . . . . .
- - - - - - Products
6Hrx = 1 3 2HI (g)
--J- -
- - - - - - - - - - - - - - - - - - - - - - - -
Reaction coordinate
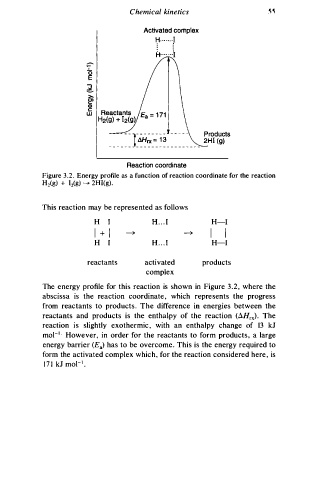
Figure 3 . 2 . Energy profile as a function of reaction coordinate for the reaction
H2(g) + 12(g) � 2Hl(g).
This reaction may be represented as follows
H I H . . I H-1
.
I + I � � I I
H I H . I H-1
.
.
reactants activated products
complex
The energy profile for this reaction is shown in Figure 3 . 2 , where the
e
abscissa is the reaction coordinat , which represents the progress
s
from reactants to product . The difference in energies between the
reactants and products is the enthalpy of the reaction (AHrx). The
reaction is slightly exothermic, with an enthalpy change of 13 kJ
mo1-1 . However, in order for the reactants to form products, a large
energy barrier (Ea) has to be overcome. This is the energy required to
form the activated complex which, for the reaction considered her , is
e
1 7 1 kJ mo1- 1 •