Page 192 - Battery Reference Book
P. 192
12/4 Metal -air cells
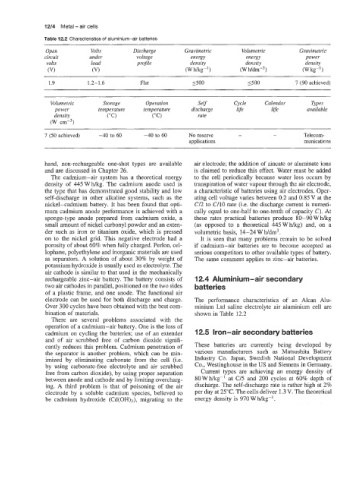
Table 12.2 Characteristics of aluminium-air batteries
Open Volts Discharge Gravimetric Volumetric Gravimetric
circuit under voltage energy energy power
volts load projile density density density
(VI (V) (Wmg-') (W h/d~~-~) (W kg- 1
1.9 1.2-1.6 Flat 1500 1500 7 (90 achieved)
Volumetric Storage Operation Self Cycle Calendar Types
power temperature temperature discharge life life available
density ("C) ("C) rate
(W ~rn-~)
7 (50 achieved) -40 to 60 -40 to 60 No reserve - - Telecom-
applications munications
hand, non-rechargeable one-shot types are available air electrode; the addition of zincate or aluminate ions
and are discussed in Chapter 26. is claimed to reduce this effect. Water must be added
The cadmium-air system has a theoretical energy to the cell periodically because water loss occurs by
density of 445 W fig. The cadmium anode used is transpiration of water vapour through the air electrode,
the type that has demonstrated good stability and low a characteristic of batteries using air electrodes. Oper-
self-discharge in other alkaline systems, such as the ating cell voltage varies between 0.2 and 0.85 V at the
nickel-cadmium battery. It has been found that opti- C/2 to C/10 rate (i.e. the discharge current is numeri-
mum cadmium anode performance is achieved with a cally equal to one-half to one-tenth of capacity c). At
sponge-type anode prepared from cadmium oxide, a these rates practical batteries produce 80-90 W hkg
small amount of nickel carbonyl powder and an exten- (as opposed to a theoretical 445 Whkg) and, on a
der such as iron or titanium oxide, which is pressed volumetric basis, 14-24 W h/dm3.
on to the nickel grid. This negative electrode had a It is seen that many problems remain to be solved
porosity of about 60% when fully charged. Perlon, cel- if cadmium-air batteries are to become accepted as
lophane, polyethylene and inorganic materials are used serious competitors to other available types of battery.
as separators. A solution of about 30% by weight of The same comment applies to zinc-air batteries.
potassium hydroxide is usually used as electrolyte. The
air cathode is similar to that used in the mechanically
rechargeable zinc-air battery. The battery consists of 12.4 Aluminium-air secondary
two air cathodes in parallel, positioned on the two sides batteries
of a plastic frame, and one anode. The functional air
electrode can be used for both discharge and charge. The performance characteristics of an Alcan Alu-
Over 300 cycles have been obtained with the best com- minium Ltd saline electrolyte air aluminium cell are
bination of materials. shown in Table 12.2
There are several problems associated with the
operation of a cadmium-air battery. One is the loss of
cadmium on cycling the batteries; use of an extender 12.5 Iron-air secondary batteries
and of air scrubbed free of carbon dioxide signifi-
cantly reduces this problem. Cadmium penetration of These batteries are currently being developed by
the separator is another problem, which can be min- various manufacturers such as Matsushita Battery
imized by eliminating carbonate from the cell (i.e. Industry Co. Japan, Swedish National Development
by using carbonate-free electrolyte and air scrubbed Co., Westinghouse in the US and Siemens in Germany.
free from carbon dioxide), by using proper separation Current types are achieving an energy density of
between anode and cathode and by limiting overcharg- 80Wh/kgp' at C/5 and 200 cycles at 60% depth of
ing. A third problem is that of poisoning of the air discharge. The self-discharge rate is rather high at 2%
electrode by a soluble cadmium species, believed to per day at 25°C. The cells deliver 1.3 V. The theoretical
be cadmium hydroxide (Cd(OH)3), migrating to the energy density is 970 W hkg-'.