Page 187 - Battery Reference Book
P. 187
Magnesium-organic electrolyte primary batteries 1113
Theoretical considerations predict that organic com-
pounds, such as meta-dinitrobenzene (m-DNB), are
especially attractive as cathode materials in primary
cells. For example, if one assumes the complete reduc-
tion of meta-dinitrobenzene, a yield of 1915 Whkg
is possible.
Considerable effort has been spent by various
battery manufacturers, including Marathon, on the
development of magnesium-magnesium perchlorate-
meta-dinitrobenzene cells. They have been made
with a cylindrical structure similar to that used with
the manganese dioxide-magnesium perchlorate dry
cells. The organic cell requires the use of a higher 0.6 L I I I J
surface area electrolyte absorbent (carbon black) to 0 8 16 24 32
be mixed with the meta-dinitrobenzene that is used Discharge time ihl
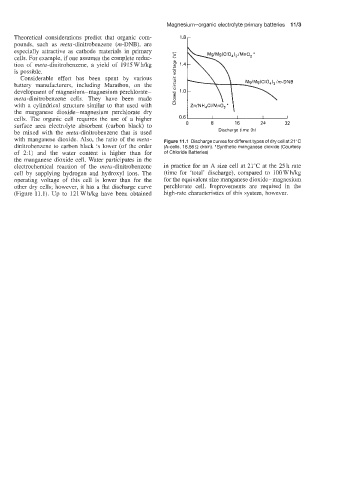
with manganese dioxide. Also, the ratio of the meta- Figure 11 .I Discharge curves for different types of dry cell at 21 ‘C
dinitrobenzene to carbon black is lower (of the order (A-cells, 16.66 S2 drain). *Synthetic manganese dioxide (Courtesy
of 2:l) and the water content is higher than for of Chloride Batteries)
the manganese dioxide cell. Water participates in the
electrochemical reaction of the meta-dinitrobenzene in practice for an A size cell at 21°C at the 25 h rate
cell by supplying hydrogen and hydroxyl ions. The (time for ‘total’ discharge), compared to IOOWhkg
operating voltage of this cell is lower than for the for the equivalent size manganese dioxide-magnesium
other dry cells; however, it has a flat discharge curve perchlorate cell. Improvements are required in the
(Figure 11.1). Up to 121 Wag have been obtained high-rate characteristics of this system, however.